Research Articles
A Comprehensive Protocol for Validating Spinal Implant Computational Models: From Theory to Clinical Translation
This article presents a detailed, step-by-step protocol for the rigorous validation of computational models used in spinal implant design and evaluation.
From Pixels to Predictions: Building Patient-Specific Finite Element Models from CT Scans for Advanced Biomedical Research
This article provides a comprehensive guide for researchers and drug development professionals on generating patient-specific finite element (FE) models from CT scans.
PRISMA Guidelines for Biomaterials Systematic Reviews: A Complete Guide to Transparent Reporting and Rigorous Synthesis
This comprehensive guide provides researchers, scientists, and drug development professionals with an in-depth understanding of applying the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines to systematic reviews...
PLGA vs. Collagen Scaffolds: Comparative Analysis of In Vivo Degradation Profiles for Tissue Engineering
This article provides a comprehensive, comparative analysis of the in vivo degradation profiles of two predominant biomaterials used in tissue engineering: synthetic Poly(lactic-co-glycolic acid) (PLGA) and natural collagen-based scaffolds.
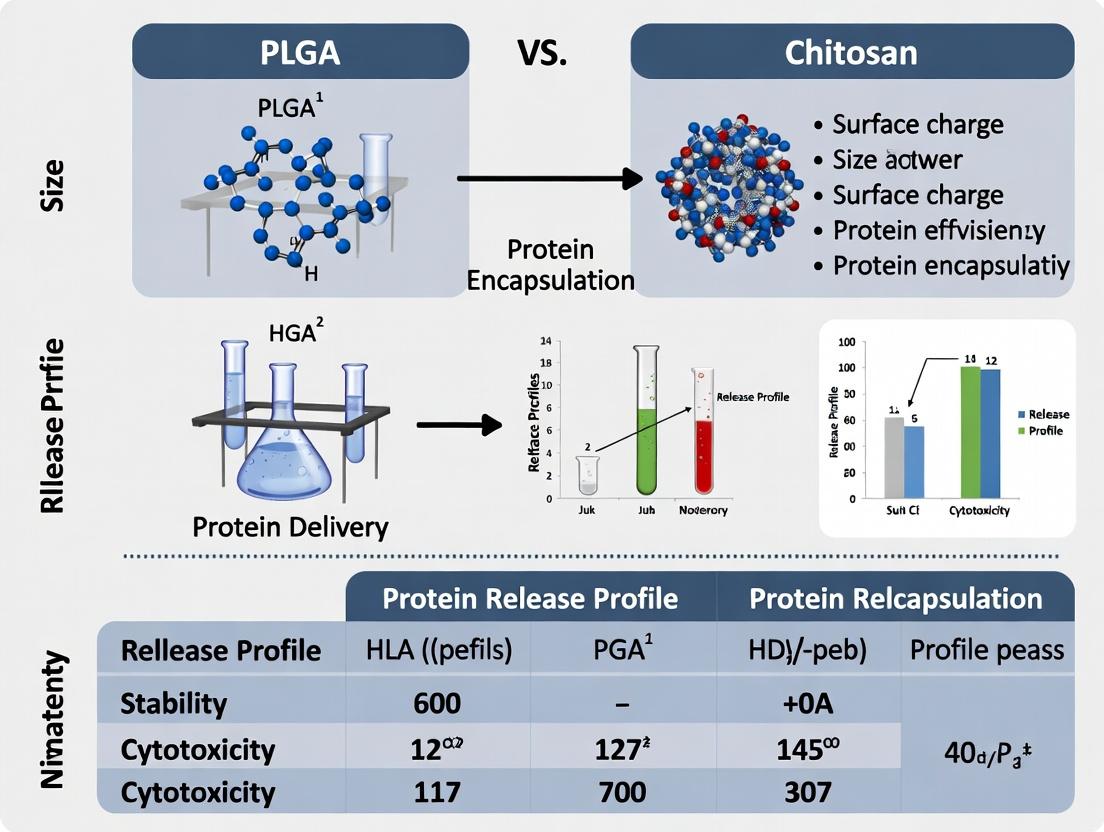
PLGA vs. Chitosan Nanoparticles: A Comparative Analysis for Protein Drug Delivery Systems
This comprehensive review analyzes PLGA and chitosan nanoparticles as leading platforms for protein delivery, addressing formulation, stability, efficacy, and clinical translation.
Mastering the PICOS Framework: A Step-by-Step Guide for Robust Biomaterial Systematic Reviews
This comprehensive guide demystifies the application of the PICOS framework for conducting systematic reviews in the rapidly evolving field of biomaterials.
PEGylation Strategies for Stealth Nanoparticles: Enhancing Circulation, Evading Immune Clearance, and Advancing Drug Delivery
This comprehensive review details the critical role of PEGylation in conferring a 'stealth' effect to therapeutic nanoparticles, enabling prolonged systemic circulation and enhanced targeting.
Solving PCR Pitfalls: A Biomaterial DNA Template Troubleshooting Guide for Researchers
This comprehensive guide addresses the unique challenges of performing PCR with DNA extracted from complex biomaterials (e.g., hydrogels, scaffolds, implants).
Extracellular Matrix Gene Detection: A Comprehensive Guide to Robust PCR Protocols for Research and Drug Development
This article provides researchers, scientists, and drug development professionals with a detailed guide to PCR protocols for detecting extracellular matrix (ECM) gene expression.
From Sequences to Signals: A Step-by-Step Guide to PCR Primer Design for Biomaterial Cellular Response Genes
This comprehensive guide provides researchers, scientists, and drug development professionals with a systematic framework for designing effective PCR primers to analyze gene expression in response to biomaterials.