Research Articles
Beyond the Mesh: A Comprehensive Guide to Understanding, Managing, and Minimizing Discretization Error in Finite Element Biomechanics
This article provides researchers, scientists, and drug development professionals with a systematic framework for addressing discretization error in finite element biomechanics.
Navigating ASTM Standards for Medical Device Materials: A Comprehensive Guide for Research and Development Professionals
This article provides a detailed exploration of ASTM International standards critical for the selection, testing, and validation of materials in medical device development.
ASTM F1635-11 Biomaterial Testing Decoded: A Complete Guide to Validating In Vitro Degradation for Medical Device R&D
This comprehensive guide provides researchers, scientists, and drug development professionals with an in-depth exploration of the ASTM F1635-11 standard for testing in vitro degradation of polymeric biomaterials.
Mastering ASTM F1635-11: The Complete Guide to Biomaterial Degradation Testing for Researchers
This comprehensive guide demystifies ASTM F1635-11, the critical standard for evaluating in vitro degradation of poly(L-lactic acid) (PLLA) resins and their fabricated forms.
The AMSTAR-2 Checklist: A Step-by-Step Guide for High-Quality Biomaterials Systematic Reviews
This comprehensive guide provides researchers, scientists, and drug development professionals with a practical framework for applying the AMSTAR-2 (A MeaSurement Tool to Assess systematic Reviews) tool to systematic reviews of...
Titanium vs. PCL in 3D Printed Bone Scaffolds: A Comparative Analysis for Biomedical Researchers
This article provides a comprehensive, evidence-based analysis of two prominent materials in 3D-printed bone tissue engineering: titanium alloys and polycaprolactone (PCL).
Bioprinting the Future: How 3D Printed Scaffolds Are Revolutionizing Tissue Engineering & Regenerative Medicine
This article provides a comprehensive analysis of 3D printed scaffolds for tissue regeneration, tailored for researchers, scientists, and drug development professionals.
Optimizing 3D Bioprinting: A Comprehensive Guide to Processing High-Viscosity Biomaterial Inks
This article provides researchers, scientists, and drug development professionals with a detailed framework for optimizing the 3D printing of high-viscosity biomaterial inks.
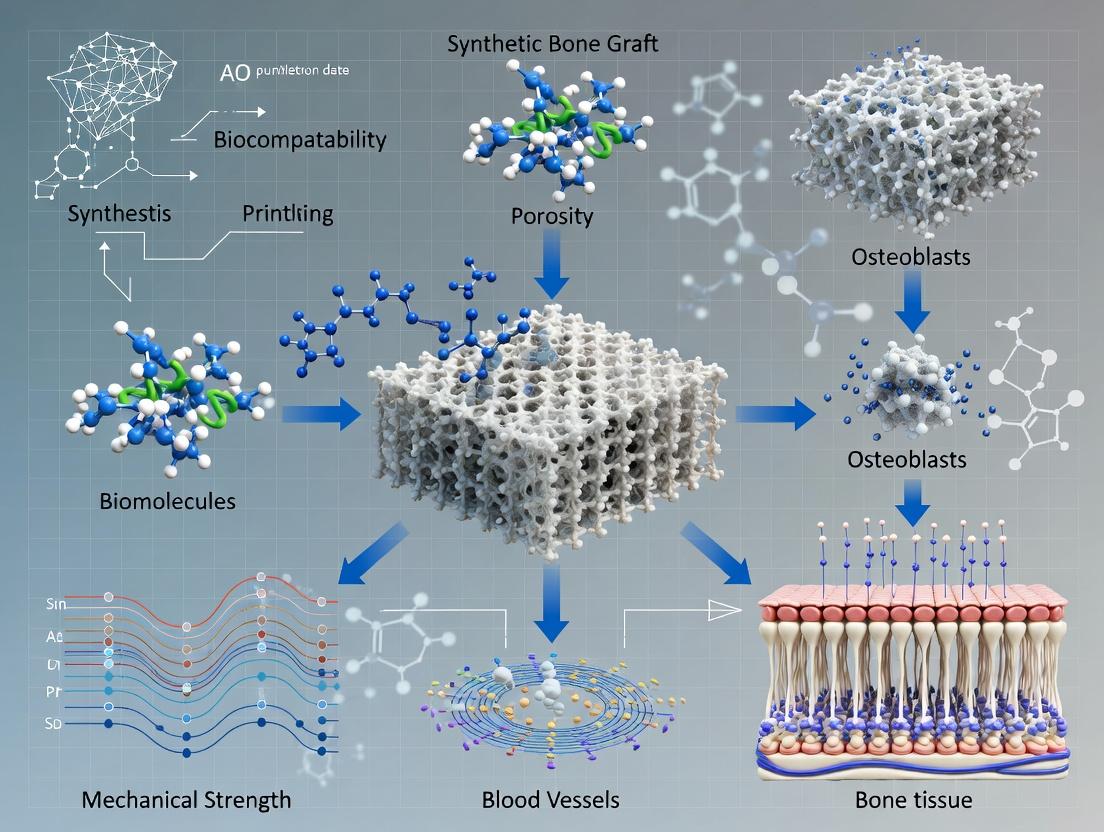
The Future of Orthopedics: 3D-Printed Synthetic Bone Graft Scaffolds for Enhanced Healing
This article provides a comprehensive overview of 3D printing technologies for synthetic bone graft scaffolds, targeting researchers and biomedical professionals.
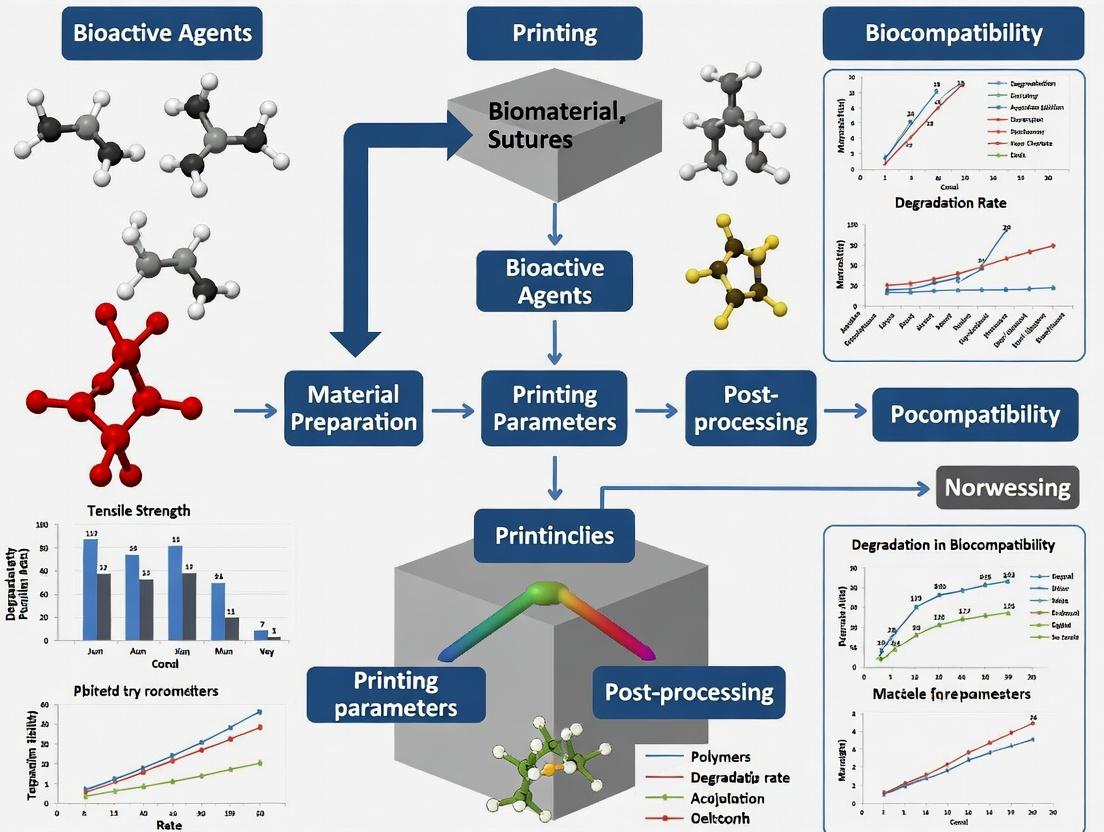
Innovations in Surgical Suture Fabrication: A Comprehensive Guide to 3D Printing Methods and Materials for Advanced Wound Closure
This article provides a detailed exploration of 3D printing methodologies for creating next-generation surgical sutures.