Strategic Approaches to Peri-Implantitis Prevention: From Microbial Pathogenesis to Advanced Biomaterials
This comprehensive review synthesizes current scientific evidence on peri-implantitis prevention strategies for researchers and drug development professionals.
Strategic Approaches to Peri-Implantitis Prevention: From Microbial Pathogenesis to Advanced Biomaterials
Abstract
This comprehensive review synthesizes current scientific evidence on peri-implantitis prevention strategies for researchers and drug development professionals. The article explores the pathological transition from microbial dysbiosis to destructive inflammation, examines novel diagnostic biomarkers in peri-implant crevicular fluid and saliva, evaluates emerging antimicrobial biomaterials and local drug delivery systems, and assesses validated clinical protocols and their long-term efficacy. By integrating foundational microbiology with applied therapeutic development, this resource provides a multidisciplinary framework for innovating preventive interventions against this complex biofilm-mediated disease.
Understanding Peri-Implantitis Pathogenesis: From Microbial Ecology to Host Immune Response
Peri-implant diseases are biofilm-associated infectious conditions affecting the tissues surrounding dental implants. The transition from health to disease involves a fundamental microbial dysbiosis, characterized by a shift in the biofilm composition from a symbiotic to a pathogenic state [1]. Understanding these microbial shifts is crucial for developing preventive strategies and targeted treatments for peri-implantitis, which affects 20-22% of patients with dental implants and represents the leading cause of implant loss [1] [2].
The ecological environment around dental implants differs significantly from that of natural teeth, both in structural anatomy and chemical composition, creating a unique niche for biofilm development [1]. Implant surfaces exhibit distinct surface energy, topography, wettability, and electrochemical charges that influence initial bacterial adhesion and biofilm formation [1]. This comprehensive technical guide provides troubleshooting resources and experimental protocols for researchers investigating these critical microbial transitions.
Frequently Asked Questions: Microbial Diagnostics & Analysis
Q1: What are the key methodological challenges in analyzing peri-implant biofilms? The complex three-dimensional structure of biofilms protects embedded microorganisms and makes complete sampling difficult. Additionally, the implant surface microstructure complicates biofilm detachment and DNA extraction. Molecular methods must overcome inhibitor resistance and achieve sufficient sensitivity to detect low-abundance pathogens [3]. For culture-based methods, sonication of removed implants has shown improved sensitivity compared to tissue cultures alone by dislodging sessile bacteria from biofilm structures [3].
Q2: How does the peri-implant microbiome differ from periodontal microbiomes? While sharing some similarities, peri-implant and periodontal microbiomes demonstrate distinct community structures. Peri-implant biofilms exhibit greater proportions of anaerobic Gram-negative bacteria in diseased states and may progress more rapidly than periodontal infections [1] [2] [4]. The peri-implant niche's structural differences create a unique environment that supports different microbial communities than those found on natural teeth [1].
Q3: What are the limitations of 16S rRNA sequencing versus shotgun metagenomics? 16S rRNA sequencing primarily provides taxonomic information with limited resolution at the species level. Shotgun metagenomics offers superior species-level identification, detects less abundant taxa, and enables functional profiling of microbial communities [4]. However, it requires higher sequencing depth and more complex bioinformatic analysis, making it more resource-intensive [5] [4].
Q4: How can researchers account for high inter-individual variability in microbiome studies? Implement within-subject controlled designs where possible, comparing diseased and healthy sites in the same individual [6] [4]. Ensure adequate sample sizes and consider longitudinal sampling to track temporal dynamics. Statistical methods like PERMANOVA can help distinguish true treatment effects from individual variations [6].
Q5: What clinical parameters best correlate with microbial dysbiosis? The sulcus bleeding index (SBI) shows strong positive correlation with microbial dysbiosis in peri-implant mucositis [7]. For peri-implantitis, increased probing depth, bleeding on probing, suppuration, and radiographic bone loss are key clinical parameters associated with pathogenic shifts [6] [5].
Quantitative Microbial Shifts: Health to Disease Transition
Table 1: Key Taxonomic Shifts in Peri-Implant Health and Disease
| Microbial Taxon | Health-Associated Abundance | Disease-Associated Abundance | Functional Role |
|---|---|---|---|
| Streptococcus | 16.91% [6] | Decreased to 13.10% [6] | Early colonizer, health-associated [1] [5] |
| Prevotella | Low prevalence [6] | Increased to 8.44% [6] | Pathogenic, anaerobic, Gram-negative [6] [7] |
| Fusobacterium | Low prevalence [6] | Increased to 16.91% [6] | Bridge colonizer, anaerobic [6] [5] |
| Neisseria | 10.06% [6] | Decreased in disease [6] | Health-associated, aerobic [6] |
| Veillonella | Prevalent in health [6] | Increased in dysbiosis [6] | Can indicate early dysbiosis [6] |
| Porphyromonas gingivalis | Absent or low [6] | Significantly increased [6] | Key periodontal pathogen [6] [5] |
| Treponema denticola | Absent or low [6] | Significantly increased [6] | Periodontal pathogen, spirochete [6] |
Table 2: Functional Pathway Alterations in Disease States
| Functional Pathway | Health Association | Disease Association | Potential Research Implications |
|---|---|---|---|
| Amino acid metabolism | Not elevated [5] | Significantly upregulated [5] | Potential therapeutic target |
| Arginine & polyamine biosynthesis | Not elevated [4] | Increased putrescine/citrulline biosynthesis [4] | Linked to inflammation |
| Protein processing (ER) | Not elevated [7] | Upregulated, correlates with SBI [7] | Biomarker potential |
| Cofactor/vitamin metabolism | Prevalent [7] | Downregulated in disease [7] | Health maintenance function |
| Purine/pyrimidine biosynthesis | Predominant in health [4] | Decreased in disease [4] | Cellular growth maintenance |
| Cell motility | Not elevated [7] | Upregulated in disease [7] | Bacterial dissemination |
Experimental Protocols for Peri-Implant Biofilm Analysis
Sample Collection and Processing
Subgingival Biofilm Collection Protocol:
- Have participants refrain from oral hygiene for 24 hours and from eating/drinking for 2 hours before sampling [4].
- Isolate sampling site with sterile cotton rolls and remove supragingival plaque to avoid contamination [6] [4].
- Collect subgingival biofilm using sterile Gracey curettes or PerioPaper strips [6] [4].
- Insert curette/paper into sulcus/pocket until slight resistance is felt; use 3 strips per implant [4].
- Pool samples into 1.5 mL DNase/RNase-free tubes containing appropriate storage buffer (e.g., 50 mM Tris-HCl, pH 7.5; 1 mM EDTA, pH 8.0; 0.5% Tween-20) [4].
- Store immediately at -80°C for subsequent DNA extraction [6] [4].
Saliva Collection Protocol (for systemic microbiome assessment):
- Collect 2 mL unstimulated saliva by drooling into 4-mL cryotube using saliva collection aid device [4].
- Add glycerol to final concentration of 20% under aseptic conditions [4].
- Store in 1 mL aliquots at -80°C [4].
DNA Extraction and Sequencing
DNA Extraction Protocol:
- Use QIAamp DNA Microbiome Kit or equivalent following manufacturer's protocols [6].
- Include negative controls to detect contamination during extraction [8].
- Verify DNA quality and quantity using spectrophotometry (e.g., Nanodrop) and fluorometry (e.g., Qubit) [6].
16S rRNA Gene Amplicon Sequencing:
- Target V3-V4 region using primers Bakt341F (5'-CCTACGGGNGGCWGCAG-3') and Bakt805R (5'-GACTACHVGGGTATCTAATCC-3') [6].
- Perform sequencing on Illumina MiSeq platform with 300 bp paired-end reads, targeting approximately 100,000 reads per sample [6].
- Include positive controls with known microbial communities to validate sequencing performance [8].
Shotgun Metagenomic Sequencing:
- Use Illumina NovaSeq platform for higher sequencing depth [7] [4].
- Target approximately 10 Gb per sample for adequate coverage [7].
- Employ library preparation kits compatible with low-biomass samples if necessary [5].
Bioinformatic Analysis Pipeline
Primary Analysis:
- Process 16S data using DADA2 pipeline for quality filtering, denoising, and chimera removal [6].
- Assign taxonomy using SILVA reference database (version 138.1) with RDP classifier [6].
- For shotgun data, use KneadData for quality control and HUMAnN2 for pathway analysis [5] [4].
Secondary Analysis:
- Calculate alpha diversity metrics (Shannon, Simpson, Chao1) [6] [7].
- Assess beta diversity using Bray-Curtis dissimilarity and Weighted Unifrac distances [6] [7].
- Perform PERMANOVA to test for group differences in community structure [6].
- Conduct differential abundance analysis with appropriate multiple testing correction [6] [5].
The Scientist's Toolkit: Essential Research Reagents & Materials
Table 3: Essential Research Reagents for Peri-Implant Biofilm Studies
| Reagent/Material | Specific Function | Example Products/Protocols | Technical Considerations |
|---|---|---|---|
| Sterile Gracey Curettes | Subgingival biofilm collection from implant sulcus | Implacare curettes [6] | Avoid cross-contamination between sites; use separate instruments |
| PerioPaper Strips | Alternative biofilm collection method | Oraflow PerioPaper Strips [4] | Particularly useful for narrow peri-implant sulci |
| DNA Extraction Kits | Microbial DNA isolation from biofilm samples | QIAamp DNA Microbiome Kit [6] | Optimized for low-biomass samples; includes host DNA depletion |
| 16S rRNA Primers | Amplification of bacterial taxonomic markers | Bakt341F/Bakt805R (V3-V4) [6] | Standardized for Illumina platforms; covers most oral taxa |
| Sequencing Kits | Library preparation and sequencing | Illumina MiSeq Reagent Kits [6] | Provide appropriate read length for 16S amplicons |
| Bioinformatic Tools | Data processing and analysis | DADA2, Phyloseq, PICRUSt2 [6] | R-based packages with specific functions for microbiome data |
| Reference Databases | Taxonomic classification | SILVA (v138.1) [6] | Regularly updated with curated oral microbiome sequences |
| Storage Buffers | Sample preservation pre-extraction | Tris-EDTA-Tween with glycerol [4] | Maintains DNA integrity during frozen storage |
Troubleshooting Common Experimental Challenges
Problem: Low DNA Yield from Biofilm Samples Solution: Concentrate samples by centrifugation after sonication (5 minutes at 0.22 W/cm²) [3]. Use specialized microbiome DNA extraction kits with enhanced lysis protocols for Gram-positive and anaerobic bacteria [6]. Include carrier RNA during extraction to improve recovery from low-biomass samples.
Problem: High Host DNA Contamination Solution: Implement host DNA depletion steps using commercial kits. Verify human DNA content with qPCR targeting human-specific genes before proceeding to sequencing. Adjust sampling technique to maximize biofilm collection while minimizing tissue contact [4].
Problem: Inconsistent Replicates in Diversity Metrics Solution: Standardize clinical sampling protocol across all operators. Ensure consistent insertion depth and pressure with curettes or paper points. Pool multiple technical replicates from the same site. Increase sample size to account for biological variability [6] [4].
Problem: Poor Correlation Between Taxonomic and Functional Data Solution: Integrate metatranscriptomic approaches (RNAseq) to assess gene expression rather than just genetic potential [5]. Ensure sufficient sequencing depth for functional profiling (≥10 Gb/sample for metagenomics). Use integrated analysis pipelines like PICRUSt2 for predicted function from 16S data [6].
Problem: Inability to Distinguish Health from Early Disease Solution: Focus on microbial community structure rather than individual taxa. Implement machine learning approaches with combined taxonomic and functional biomarkers [5]. Include longitudinal sampling to track individual transitions from health to disease [6].
Advanced Techniques: Integrated Multi-Omics Approaches
For comprehensive understanding of microbial shifts, integrated multi-omics approaches are recommended. Metatranscriptomics (RNAseq) reveals actively expressed metabolic pathways rather than just genetic potential, providing insights into community function [5]. Combined with high-resolution full-length 16S sequencing and metabolomics, this approach can identify diagnostic biomarkers with high predictive accuracy (AUC = 0.85) [5].
Key functional pathways consistently associated with peri-implantitis include:
- Amino acid metabolism and catabolism producing inflammatory metabolites [5]
- Protein processing in endoplasmic reticulum, correlating with clinical inflammation [7]
- Arginine and polyamine biosynthesis pathways [4]
These advanced functional analyses move beyond taxonomic profiling to reveal the metabolic activities driving disease progression, opening new avenues for targeted therapeutic interventions and personalized treatment approaches for peri-implant diseases.
This technical support center provides troubleshooting guides and experimental protocols for researchers investigating the host-pathogen interactions in peri-implantitis. This inflammatory condition, leading to bone loss around dental implants, involves complex interplay between oral microbiota dysbiosis and host immune dysregulation. The content is framed within the broader thesis of preventing peri-implantitis through understanding its fundamental biological mechanisms, offering scientists standardized methodologies to investigate the immune and inflammatory pathways central to this disease process.
Troubleshooting Guides: Common Experimental Challenges
FAQ: Microbial Dysbiosis Analysis
Q: What are the key pathogenic complexes in peri-implantitis and how do they differ from periodontitis-associated microbiota?
A: Peri-implantitis exhibits distinct microbial profiles compared to periodontitis. While there is some overlap with Socransky complexes, peri-implantitis biofilms show higher prevalence of specific pathogens and unique structural organization.
Table: Key Pathogenic Bacteria in Peri-Implantitis vs Periodontitis
| Bacterial Species | Role in Peri-Implantitis | Role in Periodontitis | Detection Methods |
|---|---|---|---|
| Porphyromonas gingivalis | Core microbiome component [9] [10] | Red complex member | 16S rRNA sequencing, PCR |
| Fretibacterium fastidiosum | Potential marker, more abundant in peri-implantitis [10] | Less significant | Next-generation sequencing |
| Aggregatibacter actinomycetemcomitans | Higher incidence (log OR: 4.04) [9] | Less prevalent | Microbial culture, PCR |
| Prevotella intermedia | Higher incidence (log OR: 2.28) [9] | Orange complex member | Metagenomic analysis |
| Fusobacterium nucleatum | Bridge organism in dysbiosis [10] | Similar bridging role | Fluorescence in situ hybridization |
Experimental Challenge: Inconsistent microbial profiling across studies. Solution: Standardize sample collection from deepest peri-implant pocket using sterile paper points for 30 seconds. Immediately transfer to reduced transport fluid and store at -80°C. Use mock microbial communities as positive controls for DNA extraction and sequencing.
FAQ: Immune Dysregulation Assessment
Q: Which inflammatory cytokines show most significant dysregulation in peri-implantitis and what sampling methods yield reproducible results?
A: Peri-implantitis exhibits distinct cytokine profiles compared to periodontitis, with more pronounced apical extension of inflammatory cell infiltration [9].
Table: Key Inflammatory Mediators in Peri-Implantitis
| Cytokine/Cell Type | Expression in Peri-Implantitis | Functional Role | Assessment Method |
|---|---|---|---|
| IL-1β | Significantly upregulated [9] | Pro-osteoclastogenic, amplifies inflammation | ELISA, multiplex immunoassay |
| TNF-α | Upregulated [9] | Enhances bone resorption, endothelial activation | Luminex, western blot |
| IL-17 | Elevated [9] | Links adaptive and innate immunity | Flow cytometry, mRNA quantification |
| RANKL/OPG ratio | Increased [9] | Critical for bone metabolism balance | Histomorphometry, PCR |
| Macrophages (M1 phenotype) | Increased infiltration [9] [11] | Pro-inflammatory response | Immunohistochemistry, flow cytometry |
Experimental Challenge: Variable cytokine recovery from peri-implant crevicular fluid. Solution: Use standardized peri-implant paper strip collection for 30 seconds per site. Elute in 150μL phosphate-buffered saline with protease inhibitors. Concentrate samples if using multiplex assays. Include reference standards in each assay plate.
FAQ: Titanium Particle Effects
Q: How do we experimentally model titanium particle-induced inflammation and distinguish it from bacterially-driven responses?
A: Titanium ions/particles elicit host immune response characterized by macrophage recruitment with increased IL-1β, IL-8, and IL-18 expression [9].
Troubleshooting Guide:
- Particle Generation: Use commercially available titanium particles (1-3μm diameter) at 0.1-1mg/mL concentration. Characterize with scanning electron microscopy and dynamic light scattering.
- Dosage Optimization: Perform dose-response (0.01-100μg/cm²) and time-course (6-72h) experiments in macrophage culture systems.
- Bacterial vs Particle Effects: Use TLR-specific inhibitors (TAK-242 for TLR4) to distinguish bacterial LPS responses from particle-induced sterile inflammation.
- Control Considerations: Include zirconia particles as alternative biomaterial control and polymyxin B to rule out endotoxin contamination.
Experimental Protocols: Key Methodologies
Protocol 1: Assessing NF-κB Pathway Activation in Peri-Implantitis Models
Background: NF-κB is a master inflammatory regulator of early osseointegration, controlling response to implant placement and pathogenic challenge [12].
Workflow Diagram:
Detailed Methodology:
- Cell Culture: Use RAW 264.7 macrophages or primary human macrophages cultured with titanium particles (0.1-100μg/mL) or bacterial LPS (1-100ng/mL).
- Time Course: Harvest cells at 0, 15, 30, 60, 120 minutes for phosphorylation studies and 6, 12, 24 hours for gene expression.
- Inhibitor Controls: Pre-treat with BAY-11-7082 (IKK inhibitor, 10μM) or SC-514 (IKK-2 inhibitor, 50μM) for 1 hour.
- Protein Extraction: Use RIPA buffer with protease and phosphatase inhibitors. Nuclear and cytoplasmic fractionation for translocation studies.
- Western Blotting: Primary antibodies: anti-IκBα (1:1000), anti-phospho-IκBα (1:1000), anti-NF-κB p65 (1:2000), anti-phospho-NF-κB p65 (1:1000). Normalize to β-actin or lamin B1.
- Gene Expression: Extract RNA with TRIzol, convert to cDNA. qPCR primers for IL-1β, IL-6, TNF-α, RANKL. Normalize to GAPDH.
Troubleshooting:
- High Background: Optimize antibody concentrations and increase blocking time.
- No Signal: Verify fractionation efficiency and inhibitor activity.
- Variable Response: Include positive control (TNF-α stimulation) in each experiment.
Protocol 2: Evaluating Wnt Pathway Dysregulation in Bone Healing
Background: Wnt signaling regulates later osteogenesis-related mechanisms in osseointegration, with cross-talk between inflammatory and osteogenic pathways [12].
Workflow Diagram:
Detailed Methodology:
- Cell Culture: Use MC3T3-E1 pre-osteoblasts or primary calvarial osteoblasts. Treat with Wnt3a (50-100ng/mL) in presence of titanium particles or inflammatory cytokines.
- Wnt Activation: Use CHIR99021 (GSK-3β inhibitor, 3-10μM) as positive control or DKK-1 (100ng/mL) as negative control.
- Time Points: Assess early signaling (2-8h for β-catenin localization) and late differentiation (7-21 days for mineralization).
- TOP/FOP Flash Assay: Transfert cells with TOPflash (TCF-responsive) or FOPflash (mutant control) plasmids. Measure luciferase activity 24-48h post-transfection.
- Osteogenic Markers: Alizarin Red staining for mineralization (day 21), ALP activity (day 7-14), qPCR for Runx2, Osterix, Osteocalcin.
- Inflammatory Crosstalk: Pre-treat with TNF-α (10ng/mL) or IL-1β (5ng/mL) before Wnt pathway activation.
Troubleshooting:
- Low TOP/FOP Ratio: Verify transfection efficiency with GFP control, optimize cell density.
- Variable Mineralization: Use consistent passage numbers, standardize serum lots.
- Inconsistent Effects: Test multiple Wnt ligands (Wnt3a, Wnt10b) and concentrations.
The Scientist's Toolkit: Research Reagent Solutions
Table: Essential Research Reagents for Peri-Implantitis Host-Pathogen Investigations
| Reagent Category | Specific Examples | Function/Application | Key Considerations |
|---|---|---|---|
| Pathogen-Associated Molecular Patterns | Ultrapure LPS from P. gingivalis (InvivoGen, tlrl-pglps), F. nucleatum LPS (tlrl-fnlps) | TLR2/TLR4 activation, immune response studies | Verify purity, use low passage stocks, exclude endotoxin contamination |
| Titanium Particles | Titanium (IV) oxide nanopowder (Sigma-Aldrich, 637262), commercially pure Ti particles (0.5-3μm) | Biomaterial-induced inflammation models | Characterize size distribution, sterilize by gamma irradiation, not autoclaving |
| Cytokine Inhibitors | BAY-11-7082 (IKK inhibitor), SC-514 (IKK-2 inhibitor), XAV-939 (Tankyrase/Wnt inhibitor) | Pathway-specific blockade, mechanism studies | Determine IC50 for each cell type, assess cytotoxicity in long-term cultures |
| Pathway Reporters | NF-κB luciferase reporter plasmids (pGL4.32), TOPflash/FOPflash TCF reporter systems | Pathway activation quantification | Normalize to constitutively active Renilla luciferase, optimize transfection |
| Immunoassay Kits | Luminex multiplex cytokine panels (R&D Systems), RANKL/OPG DuoSet ELISA | Multiparameter inflammatory profiling | Use matrix-matched standards, validate with spike-recovery in biological fluid |
| Cell Culture Models | RAW 264.7 macrophages, MC3T3-E1 pre-osteoblasts, primary human gingival fibroblasts | In vitro host response systems | Use low passages, authenticate regularly, mycoplasma test monthly |
Advanced Technical Considerations
Immune Cell Isolation and Characterization
For primary cell studies, isolate human peripheral blood mononuclear cells via density gradient centrifugation. Differentiate monocytes to macrophages with M-CSF (50ng/mL, 7 days). Polarize to M1 phenotype with IFN-γ (20ng/mL) + LPS (100ng/mL) or M2 with IL-4 (20ng/mL). Verify polarization with surface markers (CD80/CD86 for M1, CD206/CD163 for M2).
Titanium Particle Characterization
Beyond commercial sources, generate clinically relevant particles from actual implant materials using sterile filing and sequential sieving. Characterize with scanning electron microscopy, energy dispersive X-ray spectroscopy, and dynamic light scattering. Determine endotoxin levels with Limulus amebocyte lysate assay (<0.25 EU/mL acceptable).
Animal Model Considerations
For in vivo validation, use murine calvarial defect models with titanium implant placement. Induce peri-implantitis via silk ligature placement with P. gingivalis oral inoculation. Monitor disease progression weekly with micro-CT imaging. Include sham surgery controls and antibiotic stewardship to prevent spontaneous infection.
FAQs on Key Risk Factors for Peri-Implantitis
1. How significant is a history of periodontal disease as a risk factor for peri-implantitis? A history of periodontal disease is one of the most significant risk factors for peri-implantitis. Periodontally compromised patients have twice the risk of developing peri-implantitis compared with periodontally healthy individuals [13]. Those with a history of generalised aggressive periodontitis are at even greater risk, being 14 times more susceptible to peri-implantitis than healthy controls [13]. This strong association is partially attributed to similarities in the subgingival microbiota between diseased teeth and implants [13].
2. What is the evidence for genetic predisposition to peri-implant diseases? Research demonstrates clear intergenerational continuity in periodontal health, which serves as a proxy for peri-implant disease risk. Offspring of parents with poor periodontal health are significantly more likely to have poor periodontal health themselves [14]. After controlling for confounding factors like oral hygiene and smoking, individuals with a high-familial-risk periodontal background had a 45-64% higher risk of various periodontal disease measures compared to those from low-risk backgrounds [14]. Family history represents shared genetic variations and environmental factors that influence disease susceptibility.
3. How does smoking influence peri-implantitis risk? Smoking is a well-established risk factor that approximately doubles the risk of developing peri-implantitis compared with non-smokers [13]. Smoking impacts innate and adaptive immune responses, impairing the host's defence mechanisms and response to microbial challenges [13]. The relationship exhibits a dose-dependent pattern, with increased tissue destruction correlated with smoking intensity [13]. Emerging evidence suggests water pipes and electronic cigarettes also present significant risks, though more research is needed [13].
4. What is the relationship between diabetes and peri-implantitis? Diabetes mellitus, particularly when poorly controlled, plays a pivotal role in the progression and severity of peri-implantitis through various vascular and cellular responses that lead to enhanced tissue destruction and impaired healing [13]. The bidirectional relationship between periodontal disease and diabetes suggests that hyperglycaemia creates a pro-inflammatory state that compromises the body's ability to manage bacterial challenges around implants [13] [15].
5. Are these risk factors modifiable through intervention? Yes, several key risk factors are modifiable. Successful treatment of periodontal disease prior to implant placement can lower peri-implantitis risk [13]. Smoking cessation positively impacts periodontal health and reduces disease progression [13]. Glycaemic control in diabetic patients helps manage inflammatory responses, and adherence to regular supportive maintenance therapy significantly reduces biological complications [13] [16].
Quantitative Risk Assessment Data
Table 1: Quantified Risk Factors for Peri-Implantitis
| Risk Factor | Risk Magnitude | Population Impact | Notes |
|---|---|---|---|
| Periodontal History | 2x higher risk in periodontally compromised patients [13] | 14x higher susceptibility with aggressive periodontitis history [13] | Strongest association among patient-related factors |
| Smoking | ~2x higher risk [13] | Dose-dependent relationship [13] | Includes cigarettes, water pipes, and smokeless tobacco |
| Genetic/Familial | 45-64% increased risk [14] | RR 1.45-1.64 for various disease measures [14] | Based on parental periodontal disease history |
| Lack of Maintenance | 20% of non-compliant patients diagnosed within 5 years [13] | 86% fewer cases in compliant patients [13] | Minimum 5-6 month recall recommended |
Table 2: Microbial Associations in Peri-Implantitis
| Microbial Factor | Association Strength | Clinical Implications |
|---|---|---|
| Periodontal Pathogens | OR 15.1 for submucosal presence [13] | Specific microbiota resembling periodontal lesions |
| Microbial Diversity | Consistent marker of disease [13] | Marked diversity in peri-implantitis cases |
| Key Pathogens | P. gingivalis, A. actinomycetemcomitans, F. nucleatum [17] | Similar to periodontitis-associated flora |
Experimental Protocols for Risk Factor Investigation
Protocol 1: Microbial Analysis in Peri-Implantitis
Purpose: To characterize the submucosal microbiome around implants and identify pathogenic patterns associated with specific risk factors.
Methodology:
- Collect submucosal plaque samples from peri-implant pockets using sterile paper points
- Process samples for 16S rRNA sequencing and quantitative PCR for specific pathogens
- Analyze microbial profiles against controls from healthy implant sites
- Correlate findings with patient risk factors (smoking status, periodontal history, diabetic control)
Key Parameters:
- Presence and quantity of recognized periodontopathogens (P. gingivalis, A. actinomycetemcomitans)
- Overall microbial diversity indices
- Identification of peri-implantitis-specific microbial signatures
Protocol 2: Titanium Particle Release and Inflammation
Purpose: To investigate the release of titanium particles into peri-implant tissues and association with inflammation progression.
Methodology:
- Collect peri-implant soft tissue biopsies during surgical intervention
- Process samples for histopathological analysis and electron microscopy
- Use mass spectrometry to quantify titanium content in tissues
- Correlate titanium particle concentration with clinical inflammation markers (BOP, PD, radiologic bone loss)
Key Parameters:
- Titanium concentration in peri-implant tissues
- Histological evidence of particle-induced inflammation
- Correlation between particle load and disease severity
Visualization: Risk Factor Interrelationships in Peri-Implantitis
Diagram Title: Peri-Implantitis Risk Factor Pathways
The Scientist's Toolkit: Essential Research Reagents
Table 3: Key Research Reagents for Peri-Implantitis Investigation
| Reagent/Category | Research Application | Specific Examples |
|---|---|---|
| Microbial Analysis Tools | Characterization of peri-implant microbiome | 16S rRNA primers, qPCR assays for periodontopathogens, anaerobic culture media [13] |
| Cytokine Profiling Assays | Quantification of inflammatory response | ELISA kits for IL-1β, IL-6, TNF-α; multiplex immunoassays [13] |
| Bone Turnover Markers | Assessment of osseous metabolic activity | CTX-I (bone resorption), P1NP (bone formation) immunoassays [18] |
| Titanium Detection Kits | Quantification of implant-derived particles | ICP-MS standards, histological staining protocols [13] |
| Surface Decontamination Agents | Implant surface detoxification studies | Chlorhexidine, citric acid, hydrogen peroxide, air-powder abrasive systems [17] |
| Cell Culture Models | Host-pathogen interaction studies | Human gingival fibroblasts, osteoblast cell lines, bacterial co-culture systems [13] |
The Role of Titanium Corrosion and Particle Release in Disease Progression
Dental implants, predominantly made from titanium and its alloys, have revolutionized tooth replacement, yet biological complications like peri-implantitis threaten their long-term success. A growing body of evidence indicates that titanium corrosion and particle release play a significant role in disease progression. Titanium particles, detected in peri-implant tissues, silently prompt immune-system activation and generate pro-inflammatory responses in macrophages, T lymphocytes, and monocytes [19]. This technical support center provides researchers with targeted troubleshooting guides and experimental protocols to investigate this critical pathway and develop preventive strategies.
Troubleshooting Guide: Common Experimental Challenges
Q1: Our in vitro corrosion tests show inconsistent titanium ion release data. What factors should we control for?
A: Inconsistent ion release often stems from variable simulation of the oral environment. Key factors to control include:
- Electrolyte Composition: Use a simulated body fluid or artificial saliva that accurately reflects inorganic ion concentrations. Incorporate organic components like albumin, as proteins can influence corrosion rates [20].
- pH Dynamics: Actively monitor and control pH. The peri-implant inflammatory environment is acidic due to bacterial metabolites (e.g., H₂S from Porphyromonas gingivalis) and immune cell activity [21]. A dynamic pH system (e.g., cycling between 7.4 and 5.5) better mimics the disease state.
- Mechanical Stress: Implement a tribocorrosion setup. Titanium degradation results from the combined effect of corrosion (electrochemical) and wear (mechanical). Use a fretting or pin-on-disk apparatus to simulate micromovements at the implant-abutment interface [19] [22].
Q2: When isolating titanium particles from peri-implant tissue samples, how do we avoid contamination and ensure accurate characterization?
A: Contamination is a major challenge. Follow this workflow:
- Sample Preparation: Use ceramic or diamond knives for sectioning tissues to prevent metal introduction. Perform all procedures in a laminar flow hood.
- Particle Extraction: Digest soft tissues using high-purity enzymatic or alkaline digestants (e.g., TMAH) rather than acid, which can dissolve the particles [23].
- Characterization Triangulation: Do not rely on a single method. Combine:
- Scanning Electron Microscopy with Energy Dispersive X-ray Spectroscopy (SEM-EDS): For morphological analysis and elemental composition confirmation [19] [23].
- Laser Ablation Inductively Coupled Plasma Mass Spectrometry (LA-ICP-MS): For quantitative mapping of titanium distribution within tissue blocks [23].
- Inductively Coupled Plasma Mass Spectrometry (ICP-MS): For precise quantification of total titanium content in digested samples [23].
Q3: Our cell culture models fail to show a strong inflammatory response to commercially available titanium particles. What could be the issue?
A: The biological reactivity of titanium particles is highly dependent on their physical and chemical properties.
- Particle Size Matters: Nanoparticles (NPs) have a higher surface-area-to-volume ratio and are more biologically reactive than microparticles. Ensure you are using a size range relevant to clinical findings (nanoscale to ~45 μm) [19] [23].
- State of Particles: Commercially available "pure" titanium particles may have a stable surface oxide layer. In the inflammatory environment, this layer is compromised by reactive oxygen species (ROS) like H₂O₂. Pre-treat particles with a H₂O₂ solution to simulate this in vivo conditioning [20].
- Cell Type Selection: Use primary human macrophages or co-culture systems. Monocytes and macrophages are the primary responders. The inflammatory response is also influenced by macrophage polarization states [19] [23].
Key Mechanisms & Visual Workflows
The Vicious Cycle of Inflammation and Corrosion
Titanium particle release and peri-implantitis form a self-sustaining pathological cycle. The following diagram illustrates the key pathways and feedback loops.
Experimental Workflow for Particle Analysis
This workflow outlines a standardized protocol for detecting and analyzing titanium particles from in vitro and ex vivo samples.
Research Reagent Solutions & Essential Materials
The table below summarizes key reagents and materials used in this field of research.
Table 1: Essential Research Reagents and Materials
| Item | Function/Application | Key Considerations |
|---|---|---|
| Titanium Particles (Nanopowder) | Used for in vitro challenge models to simulate particle release. | Select size ranges (e.g., <5 μm nanoparticles) and characterize surface oxide. Pre-treatment with H₂O₂ may be necessary to simulate inflammatory conditioning [23] [20]. |
| Artificial Saliva/Simulated Body Fluid | Electrolyte for in vitro corrosion and tribocorrosion testing. | Must contain relevant ions (Cl⁻, HCO₃⁻, HPO₄²⁻). Adding proteins like albumin can increase physiological relevance [20]. |
| Hydrogen Peroxide (H₂O₂) | To simulate the oxidative burst from immune cells (neutrophils, macrophages) in an in vitro environment. | Used to pre-treat Ti particles or add to cell culture medium to mimic ROS-mediated corrosion [20]. |
| Enzymatic Digestants (e.g., Collagenase) | For extracting titanium particles from soft tissue samples without dissolving them. | Preferable to strong acids for particle recovery. Tetramethylammonium hydroxide (TMAH) is also used [23]. |
| Primary Human Macrophages | Key immune effector cells for studying the inflammatory response to Ti particles. | Response can be influenced by polarization state (M1 vs. M2). Consider using cell lines like THP-1 (differentiated) as an alternative [19] [23]. |
| ELISA/Kits for Cytokines (TNF-α, IL-1β, IL-6) | Quantification of the pro-inflammatory response triggered by Ti particles. | Essential for validating in vitro and ex vivo inflammatory models [19]. |
Advanced Experimental Protocols
Protocol: Electrochemical Corrosion Testing under Simulated Inflammation
Objective: To evaluate the corrosion resistance of titanium samples in an environment mimicking peri-implantitis.
- Materials Setup:
- Electrolyte: Phosphate Buffered Saline (PBS) with 1-5 mM H₂O₂ to simulate inflammatory ROS [20].
- Working Electrode: Titanium alloy disc (e.g., Ti-6Al-4V), polished to mirror finish.
- Reference Electrode: Saturated Calomel Electrode (SCE) or Ag/AgCl.
- Counter Electrode: Platinum wire.
- Methodology:
- Open Circuit Potential (OCP): Measure for 1 hour to establish stability.
- Potentiodynamic Polarization: Scan from -0.5 V to +1.5 V vs. OCP at a scan rate of 1 mV/s.
- Electrochemical Impedance Spectroscopy (EIS): Perform at OCP with a 10 mV amplitude perturbation, from 100 kHz to 10 mHz.
- Data Analysis:
- Calculate corrosion current density (Icorr) and corrosion potential (Ecorr) from Tafel extrapolation of polarization data.
- Fit EIS data to a modified Randles circuit to evaluate the charge transfer resistance (Rct) and stability of the passive oxide layer. A decrease in Rct indicates compromised corrosion resistance [20].
Protocol: In Vitro Macrophage Response to Titanium Particles
Objective: To quantify the pro-inflammatory cytokine release from macrophages exposed to titanium particles.
- Materials Setup:
- Cells: Primary human monocyte-derived macrophages or THP-1 cells differentiated with PMA.
- Particles: Titanium dioxide (TiO₂) nanoparticles (e.g., <100 nm). Sterilize by dry-heat or UV irradiation before use.
- Culture Conditions: Use serum-free medium during particle challenge to avoid protein-interference.
- Methodology:
- Particle Challenge: Expose cells to a concentration range of Ti particles (e.g., 10-100 μg/mL) for 24-48 hours. Include a negative control (cells only) and a positive control (e.g., LPS).
- Cell Viability Assay: Perform an MTT or WST-1 assay post-exposure to rule out cytotoxicity.
- Cytokine Analysis: Collect cell culture supernatant. Quantify TNF-α, IL-1β, and IL-6 levels using commercial ELISA kits, following the manufacturer's protocols [19] [23].
- Troubleshooting Tip: Sonication of particle stock solutions in a water bath for 30 minutes before adding to cells helps to break up agglomerates and ensure uniform exposure.
FAQ for Researchers
Q: What is the clinical evidence linking titanium particles to peri-implantitis? A: Multiple studies have consistently detected significantly higher concentrations of titanium particles in soft tissues and bone surrounding implants diagnosed with peri-implantitis compared to healthy sites [19] [22]. These particles are primarily found inside epithelial cells, macrophages, and the connective tissue, directly associating their presence with an active inflammatory lesion.
Q: Are there emerging strategies to prevent titanium particle release? A: Yes, current research focuses on surface modification technologies. Promising approaches include:
- Graphene Coatings: Vertical graphene coatings grown via Plasma-Enhanced Chemical Vapor Deposition (PECVD) show enhanced corrosion resistance, improved soft tissue sealing, and inherent antibacterial properties, creating a barrier to particle release [24].
- Alternative Materials: Zirconia-based implants are being investigated as a non-metallic alternative, completely eliminating the risk of titanium corrosion and particle release [21].
Q: How do titanium particles cause bone loss (osteolysis)? A: The mechanism is multifactorial. Titanium particles are phagocytosed by macrophages, triggering the release of pro-inflammatory cytokines (TNF-α, IL-1β, IL-6). These cytokines can directly induce the formation and activation of osteoclasts, the cells responsible for bone resorption, while simultaneously inhibiting the function of bone-forming osteoblasts, leading to a net loss of bone [19] [22] [23].
Comparative Analysis of Peri-Implantitis vs Periodontitis Pathophysiology
Microbial Etiology: Composition and Diversity
FAQ: What are the key microbiological differences between peri-implantitis and periodontitis lesions?
While both peri-implantitis and periodontitis are biofilm-mediated diseases, next-generation sequencing (NGS) reveals significant differences in their microbial composition and diversity. Peri-implantitis exhibits a more diverse microbial community with unique pathogen patterns compared to periodontitis [25] [26].
Table 1: Comparative Microbial Profiles in Disease States
| Characteristic | Periodontitis | Peri-Implantitis | Research Implications |
|---|---|---|---|
| Core Pathogens | Red complex bacteria (P. gingivalis, T. denticola, T. forsythia) [10] | Includes periodontopathogens PLUS species like Fretibacterium fastidiosum, Filifactor alocis [25] [10] | Requires broader pathogen screening beyond traditional periodontopathogens |
| Microbial Diversity | Lower diversity in lesions [26] | Higher diversity in lesions [26] [27] | Need for non-targeted sequencing approaches (16S rRNA) for complete analysis |
| Unique Species | - | Enrichment of Streptococcus parasanguinis, Streptococcus mutans, Cutibacterium acnes [27] | Potential new therapeutic targets specific to peri-implantitis |
| Community Structure | Distinct dysbiotic communities [25] | Different relative proportions of microorganisms; more complex pathogenic flora structure [25] [10] | Community-level analysis essential for understanding disease pathogenesis |
Experimental Protocol: 16S rRNA Sequencing for Microbial Profile Analysis
- Sample Collection: Using sterile paper points for 30 seconds from the deepest pocket of diseased sites [26]
- DNA Extraction: Mechanical lysis followed by commercial DNA extraction kits
- Library Preparation: Amplify V3-V4 region of 16S rRNA gene with Illumina adapter-linked primers
- Sequencing: Perform on Illumina MiSeq platform (or similar)
- Bioinformatic Analysis:
- Process sequences using QIIME2 or Mothur
- Cluster sequences into operational taxonomic units (OTUs)
- Compare diversity indices (Shannon, Chao1) between groups
- Conduct multivariate analysis for community structure differences
Host Immune Response and Inflammatory Profile
FAQ: How does the host immune response differ between peri-implantitis and periodontitis?
The host immune response in peri-implantitis demonstrates distinct characteristics from periodontitis, particularly in its cytokine profile and the potential influence of titanium particles [27].
Table 2: Inflammatory Mediators and Host Response Patterns
| Parameter | Periodontitis | Peri-Implantitis | Research Significance |
|---|---|---|---|
| Immune Dominance | Th1/Th17 response | Skewed toward Th2/Th17 dominance [27] | Different immune modulation strategies needed |
| Titanium Influence | Not applicable | Titanium particles modulate microbial community and correlate with Th17 levels [27] | Consider implant material degradation products in pathogenesis |
| Inflammatory Infiltrate | Characteristic periodontal lesion | Larger inflammatory infiltrate with greater polymorphonuclear cells [26] | More aggressive inflammatory response in peri-implant tissues |
| Functional Gene Expression | Typical periodontal pathogen activity | Heightened expression of flagella, bacterial chemotaxis, and metabolic pathway genes [27] | Different microbial virulence expression patterns |
Experimental Protocol: Crevicular Fluid Collection and Cytokine Analysis
- Site Isolation: Gently dry area with air syringe, place cotton rolls to prevent saliva contamination
- Sample Collection: Insert paper strips into crevice for 30 seconds
- Elution: Place strips in buffer solution containing protease inhibitors
- Analysis:
- Multiplex immunoassay (Luminex) for cytokine quantification
- Measure IL-1β, IL-6, IL-8, IL-10, IL-17, TNF-α
- For peri-implantitis: Include titanium level analysis via ICP-MS
- Data Interpretation: Correlate cytokine levels with clinical parameters and microbial findings
Clinical Progression and Tissue Destruction
FAQ: Why does peri-implantitis often progress more rapidly than periodontitis?
Peri-implantitis demonstrates different progression patterns compared to periodontitis due to anatomical and histological differences between teeth and implants [28] [26].
Experimental Protocol: Clinical Parameter Assessment for Longitudinal Studies
Patient Selection: Recruit based on 2017 World Workshop classification criteria:
Clinical Measurements (record at 6 sites per tooth/implant):
- Plaque Index (Silness & Löe)
- Gingival Index (Löe & Silness)
- Probing Pocket Depth
- Bleeding on Probing
- Clinical Attachment Level
- Radiographic bone loss assessment [28]
Monitoring Schedule: Baseline, 3 months, 6 months, 12 months
The Scientist's Toolkit: Essential Research Reagents and Materials
Table 3: Key Research Reagents for Peri-Implantitis/Periodontitis Investigations
| Reagent/Material | Function/Application | Specific Examples |
|---|---|---|
| Paper Points | Standardized sample collection from pockets | Sterile endodontic paper points for microbial sampling [26] |
| DNA Extraction Kits | Microbial DNA isolation for sequencing | Commercial kits (e.g., Qiagen DNeasy PowerSoil) for 16S rRNA studies [25] |
| 16S rRNA Primers | Amplification of variable regions for sequencing | V3-V4 region primers (e.g., 341F/806R) for Illumina platforms [25] |
| Multiplex Cytokine Assays | Simultaneous quantification of inflammatory mediators | Luminex-based panels for crevicular fluid analysis [27] |
| Hybenx Gel | Chemical decontamination of implant surfaces | Sulfonated phenolics gel for surface decontamination studies [29] |
| Titanium Analysis Kits | Detection of titanium particles in peri-implant tissues | ICP-MS standards for quantifying titanium release [27] |
| Sodium Bicarbonate Powder | Air polishing for implant surface decontamination | 40μm powder for air polishing in decontamination protocols [29] |
Troubleshooting Common Experimental Challenges
FAQ: Why do microbial studies show contradictory results between research groups?
Methodological heterogeneity significantly impacts results in peri-implant microbiology research [26]. Key variables include:
- Sample Collection Method: Curettes vs. paper points recover different bacterial profiles [26]
- DNA Extraction Protocol: Different yields and community representations
- Sequencing Platform and Region: V1-V3 vs. V3-V4 16S regions show variations
- Bioinformatic Pipeline: OTU clustering vs. ASV approaches affect taxonomic assignment
Solution: Implement standardized protocols across research groups and include positive controls with mock microbial communities.
FAQ: How can we improve decontamination protocols for implant surfaces?
Current research explores combined mechanical and chemical approaches:
Ten-Second Technique Protocol:
- Apply Hybenx gel for 10 seconds to contaminated surfaces
- Air polish with sodium bicarbonate powder
- Rinse with water spray for 1-2 minutes until visually clean [29]
Assessment Methods:
- Scanning electron microscopy for biofilm removal evaluation
- Energy dispersive X-ray analysis for surface element composition
- Microbiological sampling post-treatment for residual bacteria [29]
Future Research Directions and Methodological Considerations
FAQ: What are the emerging areas for investigating peri-implantitis pathophysiology?
- Metatranscriptomic Analysis: Moving beyond "who is there" to "what are they doing" by assessing functional gene expression [27]
- Titanium-Pathogen Interactions: Investigating how titanium particles influence microbial virulence and host response [27]
- Dysbiosis Indices: Developing quantitative measures of ecological imbalance specific to peri-implant diseases [10]
- Personalized Treatment Approaches: Using microbial and inflammatory profiles to tailor therapeutic interventions
Experimental Considerations:
- Always include concurrent periodontal and peri-implant sites in the same patients when possible [28] [26]
- Account for confounding factors: history of periodontitis, implant surface characteristics, prosthetic design [28]
- Consider longitudinal designs to establish causal relationships in disease progression
- Incorporate patient-reported outcome measures specific to peri-implant diseases [30]
Advanced Diagnostic Modalities and Novel Preventive Biomaterials
The table below summarizes key quantitative findings from recent studies on salivary biomarkers for periodontal and peri-implant disease detection.
Table 1: Salivary Biomarker Concentrations and Diagnostic Performance [31] [32]
| Biomarker | Concentration in Disease | Association & Diagnostic Performance | Primary Diagnostic Utility |
|---|---|---|---|
| IL-1β | 210 ± 95 pg/mL [31] [32] | Strongly associated with early gingival inflammation (p=0.01). Logistic regression coefficient: 0.25 (p=0.02). [31] [32] | Early detection of inflammatory changes. [31] [32] |
| MMP-8 | 185 ± 140 ng/mL [31] [32] | Correlated with tissue destruction (p<0.01). Higher sensitivity for advanced stages (p=0.002). Logistic regression coefficient: 0.85 (p<0.001). [31] [32] | Identifying active tissue destruction and advanced disease. [31] [32] |
Detailed Experimental Protocols
Protocol 1: Unstimulated Whole Saliva Collection and Analysis
This protocol is adapted from methodologies used to investigate IL-1β and MMP-8 in periodontal disease. [31]
1. Patient Preparation and Inclusion Criteria
- Instructions to Patients: Participants must refrain from eating, drinking (including water), smoking, chewing gum, or performing oral hygiene procedures for at least one hour before sample collection. [31]
- Inclusion Criteria: Adults (e.g., 18-65 years) with varying stages of periodontal health and disease. [31]
- Exclusion Criteria: Individuals with systemic conditions that may confound biomarker levels (e.g., diabetes, cardiovascular disorders, other inflammatory diseases), those on anti-inflammatory or immunosuppressive medication, and pregnant women. [31]
2. Sample Collection
- Time: Conduct collections between 8:00 and 10:00 a.m. to minimize effects of circadian rhythms. [31]
- Environment: Seat participants comfortably in a quiet room to reduce stress-induced variability. [31]
- Method: Collect unstimulated whole saliva by having participants passively drool into pre-cooled, sterile polypropylene containers on ice. [31]
3. Sample Processing and Analysis
- Analysis Method: Quantify biomarker levels using Enzyme-Linked Immunosorbent Assay (ELISA) kits specific for human IL-1β and MMP-8. [31]
- Data Correlation: Correlate biomarker concentrations with clinical parameters such as probing pocket depth, clinical attachment level, and bleeding on probing. [31]
Protocol 2: Peri-Implant Crevicular Fluid (PICF) Sampling
This protocol outlines the general principles for collecting PICF, a critical biofluid for peri-implantitis biomarker research.
1. Site Selection and Isolation
- Identify implant sites with varying health status (healthy, mucositis, peri-implantitis) based on clinical and radiographic examination.
- Isolate the selected implant site from oral contaminants using cotton rolls and gentle air drying. Avoid touching the gingival margin.
2. Fluid Collection
- Method: Place standardized paper strips (e.g., Periopaper) at the entrance of the peri-implant crevice for a defined period (e.g., 30 seconds). Avoid forcing the strip deeply into the sulcus.
- Control: Sample a contralateral healthy tooth or a healthy implant site as a control.
- Handling: Strips containing visible blood contamination should be discarded. Place the used strips into pre-chilled microcentrifuge tubes containing a suitable elution buffer.
3. Sample Elution and Storage
- Centrifuge the tubes to elute the PICF from the strips into the buffer.
- Aliquot the eluent and store at -80°C until further analysis.
Frequently Asked Questions (FAQs) & Troubleshooting
Q1: In saliva-based biomarker studies, what are the critical pre-analytical factors that most impact result variability, and how can I control them?
- Problem: High variability in biomarker concentrations between samples.
- Solution:
- Standardize Timing: Collect samples at the same time of day (e.g., morning) to account for circadian rhythms. [31]
- Control Diet and Oral Hygiene: Instruct patients to fast and abstain from oral hygiene for at least one hour pre-collection. [31]
- Specify Collection Method: Use unstimulated whole saliva collection for baseline inflammatory markers, as stimulation can alter composition. [31]
Q2: My PICF samples yield very low fluid volumes. How can I ensure sufficient analyte for multiplex analysis?
- Problem: Insufficient sample volume for detecting multiple biomarkers.
- Solution:
- Pool Samples: For a given patient and clinical condition, pool PICF from multiple similar sites (e.g., all sites with probing depth >5mm).
- Use High-Sensitivity Assays: Employ detection methods with high sensitivity, such as multiplex ELISA or electrochemiluminescence assays, which require smaller sample volumes.
- Optimize Elution: Minimize the volume of elution buffer used to concentrate the sample.
Q3: What is the key difference between a biomarker for early inflammation versus tissue destruction, and how does this guide interpretation?
- Problem: Misinterpreting the clinical significance of different biomarker signals.
- Solution: Biomarkers play distinct roles in the disease process. IL-1β is a pro-inflammatory cytokine primarily released by immune cells and is a strong indicator of early, active inflammation. [31] In contrast, MMP-8 is an enzyme that degrades collagen in the extracellular matrix, making it a direct marker of active tissue destruction. [31] An elevated IL-1β level in an otherwise clinically healthy site may signal risk for future breakdown, while a high MMP-8 level often confirms active, ongoing destruction in advanced disease. [31]
Q4: How can I frame my biomarker research within the broader context of preventing peri-implantitis?
- Problem: Connecting laboratory findings to clinical preventive strategies.
- Solution: Position your work within the established risk factors and prevention paradigms.
- Risk Assessment: Emphasize that a history of periodontitis is a major risk factor for peri-implantitis. [33] Your research could identify biomarkers that stratify this risk more precisely.
- Preventive Strategy: Link your findings to the need for long-term maintenance programs. [33] Propose that your biomarkers could be used to monitor implant health during these recall visits, allowing for early intervention before irreversible bone loss occurs.
- Novel Interventions: Reference emerging strategies, such as coating implant surfaces with antimicrobial peptides (AMPs) to prevent bacterial colonization. [34] Suggest that your biomarker panel could be used to evaluate the efficacy of such novel coatings in clinical trials.
The Scientist's Toolkit: Research Reagent Solutions
Table 2: Essential Materials for Biomarker Research in Peri-Implant Diseases
| Item | Function/Application | Examples & Notes |
|---|---|---|
| Sterile Saliva Collection Tubes | Collection and initial storage of unstimulated whole saliva. [31] | Pre-cooled polypropylene tubes; ensure they are non-cytotoxic. |
| Periopaper Strips | Standardized collection of Peri-Implant Crevicular Fluid (PICF). | Oraphlow; volume measured with a pericrometer. |
| ELISA Kits | Quantification of specific biomarkers (e.g., IL-1β, MMP-8, RANKL) in saliva and PICF eluent. [31] | Human-specific DuoSet ELISA Kits (R&D Systems); multiplex arrays can conserve sample. |
| Protein Elution Buffer | Extraction of proteins and biomarkers from collection strips. | Buffer containing protease inhibitors to prevent protein degradation. |
| Microcentrifuge Tubes | Sample storage and processing. | Use low-protein-binding tubes to maximize recovery. |
Experimental Workflow for Biomarker Analysis
The diagram below outlines the core workflow for a biomarker discovery and validation study in peri-implant diseases.
Biomarker Analysis Workflow
Metatranscriptomic Approaches for Identifying Functional Biomarkers in Biofilms
Frequently Asked Questions (FAQs)
Q1: What is the primary advantage of using metatranscriptomics over metagenomics for studying peri-implantitis biofilms?
Metatranscriptomics analyzes the total RNA extracted from a biofilm sample, revealing the actively expressed genes and functional pathways of the microbial community at the time of sampling. In contrast, metagenomics sequences DNA, which provides information about the taxonomic composition and the potential functional capacity of the community, but not what is actively being expressed. For peri-implantitis, this is crucial because it identifies which virulence factors, metabolic pathways, and stress responses are actually being utilized by the biofilm, offering direct insight into the mechanisms driving disease pathogenesis [5] [35]. Studies have shown that the taxonomic composition (DNA) and functional activity (RNA) can be discordant, emphasizing the need for metatranscriptomics to understand the true pathological state [36].
Q2: Why is my metatranscriptomic data dominated by host and ribosomal RNA, and how can I improve microbial mRNA yield?
This is a common challenge when working with clinical biofilm samples, which often have low microbial biomass.
- Host Contamination: Biofilm samples from peri-implant sites are inevitably contaminated with host cells (e.g., gingival crevicular fluid, blood).
- High Ribosomal RNA (rRNA): In bacterial cells, rRNA can constitute over 95% of the total RNA, overwhelming the mRNA signal.
Solutions and Troubleshooting:
- Biomass Enrichment: During sample collection, carefully remove supragingival plaque and use paper points specifically designed for subgingival biofilm sampling to minimize host fluid uptake [4].
- Probe-Based rRNA Depletion: Use commercial kits that contain probes to selectively remove both host and bacterial rRNA. This is more effective for complex communities than poly-A enrichment (which only selects eukaryotic mRNA).
- Optimized Lysis Protocols: Use rigorous mechanical lysis methods (e.g., bead beating) to ensure robust extraction from tough Gram-negative bacterial cell walls, which are common in dysbiotic biofilms [37] [38].
Q3: What are the key functional pathways I should focus on when analyzing metatranscriptomic data from peri-implantitis?
Research consistently implicates several upregulated functional pathways in peri-implantitis biofilms compared to healthy implants. The table below summarizes key pathways and their suspected role in pathogenesis.
Table 1: Key Dysbiotic Functional Pathways in Peri-Implantitis
| Functional Pathway | Biological Function | Association with Peri-Implantitis |
|---|---|---|
| LPS Biosynthesis | Major component of the outer membrane of Gram-negative bacteria; a potent endotoxin. | Significantly upregulated; drives host inflammatory response and tissue destruction [39]. |
| Amino Acid Metabolism (e.g., Arginine, Tryptophan degradation) | Breakdown of host proteins and peptides for nutrition. | Creates a proteolytic environment; produces toxic metabolites like ammonia and hydrogen sulfide [5] [39]. |
| Bacterial Secretion Systems (e.g., Type IV) | Delivery of virulence factors directly into host cells. | Highly active; facilitates host cell manipulation and invasion [39]. |
| Iron Acquisition Systems | Scavenging essential iron from the host environment. | Upregulated; critical for survival and virulence of pathogens in the iron-limited host environment [40] [39]. |
| Putrescine & Citrulline Biosynthesis | Production of specific polyamines and amino acid derivatives. | Correlated with diseased implants; may modulate host immunity and biofilm ecology [4]. |
Q4: How can I validate that my metatranscriptomic findings are biologically relevant?
- Correlation with Metagenomics: Compare your RNA data with DNA-based metagenomic data from the same sample. This confirms that the active functions are not just an artifact of a few highly transcribed genes in minor populations [36].
- Integration with Clinical Parameters: Statistically correlate the expression levels of key genes or pathways with clinical parameters like probing depth, bleeding on probing, and radiographic bone loss. This links molecular findings to disease severity [5] [4].
- Culture-Based Validation: Isolate key pathogenic species identified in the study (e.g., Porphyromonas gingivalis) and use techniques like RT-qPCR to validate the high expression of specific virulence genes (e.g., fimbrillin genes) in vitro [37].
Troubleshooting Guides
Issue: Low Sequencing Library Complexity or High Replicate Variability
Potential Causes and Solutions:
Cause 1: Inconsistent Biofilm Maturation.
- Solution: Use a standardized in vitro biofilm model where possible. For clinical samples, strictly adhere to clinical criteria for patient selection and sample the deepest pocket depth consistently. In vitro models like constant depth film fermenters (CDFF) can provide highly reproducible biofilms by mimicking the oral environment with constant nutrient flow and shear force [38].
Cause 2: Inefficient RNA Extraction and Contaminants.
- Solution:
- Lysis: Ensure complete lysis by combining enzymatic (e.g., lysozyme) and mechanical (e.g., bead beating) methods. Visually inspect the lysis buffer for clarity post-treatment.
- Inhibition: Check for contaminants like humic acids or polysaccharides using a spectrophotometer (e.g., Nanodrop). An A260/A230 ratio below 2.0 may indicate contamination. Perform additional clean-up steps with column-based kits if necessary.
- Protocol: Use a dedicated, optimized protocol for complex biofilms. For example, the protocol described by (9) for oral biofilms uses RNAProtect for immediate stabilization, followed by bead beating and silica-membrane column purification [36].
- Solution:
Issue: Inconclusive Differential Gene Expression Analysis
Potential Causes and Solutions:
Cause 1: Inadequate Sample Size or Statistical Power.
- Solution: Perform a power analysis before the study if possible. For clinical studies, a sample size of 10 per group (healthy vs. disease) has been used successfully to identify strong transcriptional signatures, but larger cohorts are always beneficial [39]. Use multivariate statistical methods like PERMANOVA that are robust for community-level data.
Cause 2: Over-reliance on Taxonomic Abundance from RNA data.
- Solution: Remember that mRNA read counts are a function of both a microbe's abundance and its transcriptional activity. Normalize your metatranscriptomic data using metagenomic sequencing data from the same sample (genome-normalized transcriptomes) to distinguish true per-cell activity from population abundance effects [36].
The Scientist's Toolkit: Research Reagent Solutions
Table 2: Essential Reagents and Kits for Metatranscriptomic Workflows
| Item | Function / Application | Examples / Considerations |
|---|---|---|
| RNA Stabilization Reagent | Immediately stabilizes RNA in situ at the collection site, preserving the authentic transcriptional profile and preventing degradation. | RNAProtect (QIAGEN) [36]. Critical for clinical samples with a lag time to the lab. |
| Mechanical Lysis System | Breaks open tough bacterial cell walls (e.g., Gram-positive and Gram-negative) to ensure representative RNA extraction from all community members. | Mini-BeadBeater with 0.1mm glass or zirconia beads [37] [36]. |
| rRNA Depletion Kit | Selectively removes abundant ribosomal RNA to enrich for messenger RNA, dramatically increasing sequencing depth for informative transcripts. | Probe-based kits designed for "microbial rRNA depletion" (e.g., from Illumina, Thermo Fisher). More effective for bacteria than poly-A enrichment. |
| cDNA Synthesis & Library Prep Kit | Converts fragmented mRNA into a sequencing-ready library of cDNA fragments with adapters. | Kits compatible with low-input RNA and dual-index unique molecular identifiers (UMIs) to reduce PCR duplicate bias. |
| Reference Genome Database | A curated, comprehensive database for accurate taxonomic assignment and functional annotation of sequencing reads. | Human Oral Microbiome Database (HOMD), Virulence Factor Database (VFDB) [40], KEGG, GO [39]. Using a tailored database improves resolution. |
Experimental Workflow & Data Analysis Diagrams
Diagram 1: From Sample to Insight - Metatranscriptomic Workflow
The following diagram illustrates the end-to-end workflow for a metatranscriptomics study, from sample collection through data analysis and validation.
Diagram 2: Data Analysis Pipeline Logic
This diagram outlines the core logical steps and tools used in the bioinformatic analysis of metatranscriptomic data.
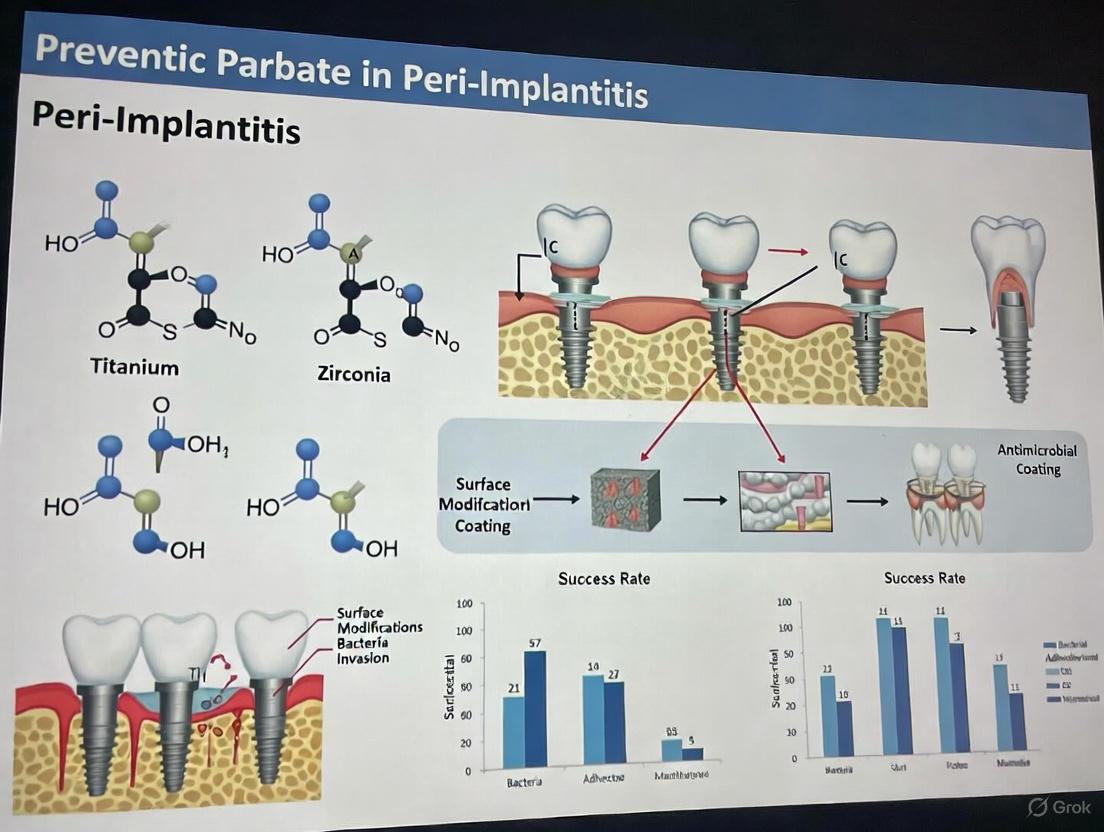
This technical support center is designed as a resource for researchers and scientists developing antimicrobial peptide (AMP) coatings for titanium implants to prevent peri-implantitis. The following guides and FAQs address common experimental challenges, provide detailed protocols, and present key quantitative findings from recent studies to support your investigative work.
Frequently Asked Questions (FAQs) and Troubleshooting Guides
FAQ 1: What is the optimal number of AMP coating layers for effective bacterial inhibition?
Answer: Research indicates that a 9-layer AMP coating provides significantly superior antimicrobial activity compared to 3-layer and 6-layer configurations.
- Evidence: A 2024 study demonstrated that 9-layer AMP-coated titanium discs exhibited "greatly higher" antimicrobial activity against Porphyromonas gingivalis and Staphylococcus aureus compared to discs with fewer layers (P < 0.05) [41].
- Troubleshooting Tip: If encountering inconsistent results, verify layer uniformity using X-ray photoelectron spectroscopy (XPS) to confirm successful deposition of each layer [41].
FAQ 2: How can I achieve sustained AMP release for long-term protection?
Answer: Implementing a layer-by-layer (LBL) assembly technique with appropriate barrier layers can extend release duration from days to weeks.
- Evidence: Studies utilizing chitosan and hyaluronic acid as polyelectrolyte layers in LBL assembly demonstrated controlled AMP release over 30 days [41] [42].
- Advanced Solution: Incorporating a phospholipid (POPC) top layer as a diffusion barrier further sustains release kinetics, fitting a first-order release model for prolonged activity [43].
- Troubleshooting: If experiencing rapid release burst, increase the number of barrier layers or incorporate cross-linking agents to strengthen the coating matrix.
FAQ 3: How do I evaluate coating stability and durability?
Answer: Utilize these standardized tests:
- Contact angle measurements to assess surface wettability changes after coating [41] [42]
- Thermogravimetric analysis (TGA) to verify coating stability under sterilization conditions [44]
- Ultrasonic vibration testing in various solvents to evaluate adhesion strength [42]
FAQ 4: What are the primary cytotoxicity considerations for AMP coatings?
Answer: Comprehensive biocompatibility assessment should include:
- Cytotoxicity testing with human dermal fibroblasts or osteoblast cell lines using CCK-8 assays [45]
- Hemolysis assays to evaluate erythrocyte damage potential [42]
- In vivo immunotoxicity assessment measuring serum immunoglobulin levels [42]
- Note: Studies with Tet213 and HHC-36 AMPs showed minimal cytotoxicity and hemolysis at effective antimicrobial concentrations [42] [45].
Quantitative Efficacy Data
Table 1: Antibacterial Efficacy of AMP-Coated Titanium Discs with Varying Layers
| Coating Layers | Bacterial Strain | Reduction in Growth | Early Biofilm Formation | Significance |
|---|---|---|---|---|
| 3-layer AMP | P. gingivalis ATCC BAA-308 | Moderate | Moderate inhibition | P < 0.05 |
| 3-layer AMP | S. aureus ATCC 25923 | Moderate | Moderate inhibition | P < 0.05 |
| 6-layer AMP | P. gingivalis ATCC BAA-308 | High | Significant inhibition | P < 0.05 |
| 6-layer AMP | S. aureus ATCC 25923 | High | Significant inhibition | P < 0.05 |
| 9-layer AMP | P. gingivalis ATCC BAA-308 | Very High | Nearly complete inhibition | P < 0.05 |
| 9-layer AMP | S. aureus ATCC 25923 | Very High | Nearly complete inhibition | P < 0.05 |
| Uncoated titanium | Both strains | No inhibition | No inhibition | Reference |
Table 2: Release Kinetics of AMP from Coated Titanium Discs in PBS (pH 7.4) at 37°C
| Time Point | 3-Layer Coating | 6-Layer Coating | 9-Layer Coating |
|---|---|---|---|
| 1 hour | Initial burst release | Initial burst release | Initial burst release |
| 12 hours | Rapid release phase | Rapid release phase | Rapid release phase |
| 1 day | Peak concentration | Peak concentration | Peak concentration |
| 5 days | Declining release | Sustained release | Sustained release |
| 10 days | Minimal release | Continued release | Continued release |
| 15 days | Depleted | Declining release | Sustained release |
| 20 days | - | Minimal release | Continued release |
| 25 days | - | - | Declining release |
| 30 days | - | - | Detectable release |
Detailed Experimental Protocols
Protocol 1: Layer-by-Layer AMP Coating on Titanium Substrates
Materials Required:
- Polished titanium discs (10×10×1 mm)
- Chitosan solution (5 mg/mL in 0.2% acetic acid)
- Hyaluronic acid solution (0.5 mg/mL in distilled water)
- AMP solution (1 mg/mL in distilled water) [42]
Procedure:
- Surface Preparation: Clean titanium discs sequentially in acetone, ethanol, and deionized water using ultrasonic agitation for 15 minutes each [42].
- Precursor Layer: Immerse discs in chitosan solution for 30 minutes to establish a positively charged foundation layer.
- Rinse: Rinse three times with sterile water to remove unbound chitosan.
- Polyanion Layer: Transfer discs to hyaluronic acid solution for 5 minutes to deposit a negatively charged layer.
- Rinse: Again, rinse three times with sterile water.
- AMP Layer: Immerse discs in AMP solution for 5 minutes for the first AMP layer deposition.
- Rinse: Rinse three times with sterile water.
- Repetition: Repeat steps 4-7 until desired number of layers is achieved (typically 3, 6, or 9 layers) [41].
- Drying: Air-dry coated discs under nitrogen flow and store in sterile conditions until use.
Quality Control:
- Verify coating success through water contact angle measurements (should show increased hydrophilicity) [41]
- Confirm surface chemical composition using XPS [41]
Protocol 2: In Vitro Antimicrobial Activity Assessment
Materials Required:
- Bacterial strains (P. gingivalis ATCC BAA-308 and S. aureus ATCC 25923) [41]
- Brain Heart Infusion (BHI) broth and agar
- Anaerobic chamber (for P. gingivalis)
- Sterile culture plates
Procedure:
- Bacterial Preparation: Culture bacteria in BHI broth until reaching log growth phase (typically 5 days for P. gingivalis, 5 hours for S. aureus) [41].
- Standardization: Adjust bacterial suspension to approximately 1×10⁸ to 1×10⁹ CFU/mL using turbidimetry.
- Inoculation: Apply 0.3 mL of bacterial suspension to coated and uncoated titanium discs in culture plates containing 3 mL BHI broth.
- Incubation:
- For P. gingivalis: Incubate anaerobically at 37°C for 5 days
- For S. aureus: Incubate aerobically at 37°C for 24 hours
- Analysis:
- Streak samples onto BHI agar plates using cotton swabs
- Incubate again under appropriate conditions
- Count resulting colonies to quantify bacterial viability
Troubleshooting:
- If contamination occurs, verify sterilization protocols for titanium discs (recommended: UV sterilization overnight) [41]
- If bacterial growth is inconsistent between replicates, standardize inoculation density using optical density measurements rather than turbidity alone
Signaling Pathways and Experimental Workflows
Diagram Title: Layer-by-Layer Coating Workflow
The Scientist's Toolkit: Research Reagent Solutions
Table 3: Essential Materials for AMP Coating Research
| Reagent/Material | Function/Purpose | Example Specifications |
|---|---|---|
| Titanium substrates | Implant simulation | 10×10×1 mm plates, commercially pure titanium [42] |
| Chitosan | Polyelectrolyte precursor layer | 5 mg/mL in 0.2% acetic acid, molecular weight: 50-190 kDa [42] |
| Hyaluronic acid | Polyelectrolyte counterion layer | 0.5 mg/mL in distilled water [42] |
| Antimicrobial peptides | Active antimicrobial agent | Tet213 (KRWWKWWRRC), HHC-36, or custom sequences [42] [45] |
| Sulfo-SMPB | Crosslinking agent | Sulfosuccinimidyl-4-(p-maleimidophenyl) butyrate [42] |
| Type I collagen | AMP composite matrix | Combined with AMPs via Sulfo-SMPB to form CSN-AMP [41] |
| Phosphate Buffered Saline (PBS) | Release kinetics medium | pH 7.4, for simulating physiological conditions [41] |
| Poly D,L-lactic acid (PDLLA) | Sustained-release polymer matrix | 2g in 40mL ethyl acetate for HHC-36 encapsulation [45] |
| Poly lactic-co-glycolic acid (PLGA) | Alternative sustained-release matrix | Forms nanoparticles with HHC-36 [45] |
Local Drug Delivery Systems for Sustained Antimicrobial Protection
Key Concepts and Reagent Solutions
Local drug delivery systems (LDDS) provide a targeted approach for sustained antimicrobial release directly at the site of infection, such as peri-implant pockets. These systems maintain effective drug concentrations while minimizing systemic side effects, making them crucial for preventing and managing peri-implantitis [46]. The table below summarizes key research reagents and their functions in developing these systems.
Table: Essential Research Reagents for Local Antimicrobial Delivery Systems
| Material Category | Specific Examples | Primary Function in LDDS |
|---|---|---|
| Biopolymers (Natural) | Gelatin, Chitosan, Collagen [46] | Biodegradable matrix for controlled drug release; offers biocompatibility. |
| Biopolymers (Synthetic) | Poly(D,L-lactide-co-glycolide) (PLGA), Polyvinyl Alcohol (PVA) [46] | Provides a predictable degradation rate and drug release profile (e.g., zero-order kinetics). |
| Antimicrobial Agents | Chlorhexidine Gluconate, Tetracycline, Nystatin, Amphotericin B [46] [47] | Active pharmaceutical ingredient to suppress or eliminate pathogenic biofilms. |
| Phospholipids | Phosphatidylcholine (PC) [47] | Key component for forming liposomal delivery systems to encapsulate drugs. |
| Carrier Polymers | Polyvinylpyrrolidone (PVP) [47] | Used in electrospinning and fiber formation to create amphiphilic composite nanofibers. |
Troubleshooting Common Experimental Challenges
FAQ 1: How can I modify the drug release profile from a polymeric film to extend its duration beyond 24 hours?
- Challenge: Initial formulations release the drug too rapidly.
- Solution: Consider using a composite multi-layer film structure. For instance, a chitosan/PLGA/chitosan film has been shown to prolong the release of ipriflavone for up to 20 days, compared to a single-layer film [46]. The different polymer layers act as sequential diffusion barriers, enabling more controlled, zero-order release kinetics ideal for controlled delivery devices.
FAQ 2: What could cause low encapsulation efficiency of an antimicrobial agent in liposomes formed via electrospun nanofiber templates?
- Challenge: The final liposome dispersion contains a lower-than-expected drug load.
- Investigation & Resolution:
- Verify Drug-Polymer Compatibility: During the electrospinning process, ensure the drug is uniformly dispersed within the polymer solution. Incompatibility can lead to phase separation and drug expulsion.
- Optimize Hydration Parameters: The hydration step of the phospholipid-laden nanofibers is critical. Agitation speed, temperature, and volume of the hydrating medium can affect liposome formation and drug retention. This method, while faster, may yield different efficiencies (14.9-28.1%) compared to the conventional film-hydration method (22.0-77.1%) [47].
FAQ 3: How do I address the inherent toxicity of a potent antimicrobial like Amphotericin B in a local delivery formulation?
- Challenge: The drug is effective but causes undesirable local tissue toxicity.
- Solution: Encapsulate the drug within a biodegradable polymer. Research shows that loading polyene antifungals like Amphotericin B and Nystatin into medium-chain-length polyhydroxyalkanoate (mcl-PHA) microspheres completely eliminated toxicity and teratogenicity in a zebrafish model, even at high doses, while successfully eradicating C. albicans infection [47]. The polymer matrix acts as a barrier, modulating the drug's release and interaction with surrounding tissues.
Standard Experimental Protocol: Preparation of Electrospun Nanofiber-Based Liposomes
This protocol details the synthesis of antibiotic-loaded liposomes using electrospun amphiphilic nanofibers as templates, adapted from research findings [47].
Methodology:
- Solution Preparation: Dissolve the polymer (e.g., Polyvinylpyrrolidone, PVP) and phospholipid (e.g., Phosphatidylcholine, PC) in a suitable solvent such as ethanol. Incorporate the model antibacterial drug (e.g., Chloramphenicol, CAM) at the desired concentration into this solution under constant stirring to ensure a homogeneous mixture.
- Electrospinning: Load the prepared solution into a syringe fitted with a metallic needle. Apply a high voltage (typically between 10-25 kV) to the needle while controlling the solution flow rate (e.g., 1.0 mL/h). Collect the resulting composite drug-loaded nanofibers on a grounded collector drum placed at a fixed distance (e.g., 15 cm).
- Fiber Characterization: Analyze the obtained nanofibers using scanning electron microscopy (SEM) to determine the average fiber diameter and morphology.
- Liposome Formation via Hydration: Place a measured section of the nanofiber mat into an aqueous buffer. Hydration of the phospholipids integrated within the fibers will occur spontaneously within seconds, leading to the in-situ formation of liposomes in the dispersion.
- Post-Processing (Optional): To achieve a more uniform liposome size distribution, the resulting dispersion can be subjected to extrusion through polycarbonate membranes of defined pore sizes.
- Characterization and Analysis:
- Particle Size: Determine the hydrodynamic diameter of the liposomes using dynamic light scattering (DLS).
- Encapsulation Efficiency (EE): Separate the free (unencapsulated) drug from the liposomes using centrifugation or dialysis. Calculate the EE using the formula:
EE (%) = (Amount of drug in liposomes / Total amount of drug used) × 100. - Drug Release: Place the liposome dispersion in a dialysis bag immersed in a release buffer. Agitate at a constant temperature. At predetermined time points, withdraw samples from the external buffer and analyze the drug concentration via HPLC or UV-Vis spectroscopy to generate a release profile.
The workflow for developing and evaluating these systems is summarized in the following diagram.
The performance of different local delivery systems can be evaluated based on key pharmacokinetic and efficacy parameters. The table below consolidates quantitative data from research studies.
Table: Performance Metrics of Local Drug Delivery Systems
| System Type | Active Ingredient | Key Performance Metric | Reported Value / Outcome | Reference |
|---|---|---|---|---|
| Biodegradable Chip | Chlorhexidine Gluconate (2.5 mg) | Probing Depth Reduction | Significant improvement vs. scaling/root planing alone | [46] |
| Composite Multi-layer Film | Iprivlavone | Drug Release Duration | Prolonged release for up to 20 days | [46] |
| mcl-PHA Microspheres | Amphotericin B (AmB) | In-vivo Toxicity (Zebrafish) | Toxicity eliminated at 100x MIC dose | [47] |
| Fiber-Hydration Liposomes | Chloramphenicol (CAM) | Encapsulation Efficiency (EE) | 14.9% to 28.1% (varies with concentration) | [47] |
| Film-Hydration Liposomes | Chloramphenicol (CAM) | Encapsulation Efficiency (EE) | 22.0% to 77.1% (varies with concentration/extrusion) | [47] |
Electrolytic decontamination represents a technologically advanced approach to eradicating pathogenic microorganisms, with growing importance in biomedical fields such as dental implant therapy. This process utilizes electrochemical principles to generate potent disinfecting agents directly at the site of contamination, offering a targeted and controllable method for eliminating biofilm and bacteria associated with peri-implant diseases [48] [49]. The core advantage of this technology lies in its ability to perform on-demand disinfection without requiring the storage and handling of hazardous chemicals, making it particularly suitable for clinical environments where precision and safety are paramount [49].
Within dental implantology, electrolytic cleaning has emerged as a promising strategy for managing peri-implantitis, a destructive inflammatory disease affecting the soft and hard tissues surrounding dental implants. With peri-implantitis affecting approximately 19.53% of patients at the patient level and 12.53% at the implant level, effective decontamination methods are critically needed [50]. Traditional non-surgical decontamination approaches often demonstrate limited efficacy in obtaining disease resolution, creating an urgent need for advanced therapeutic options [50]. Electrolytic decontamination addresses this need through its unique mechanism of action that simultaneously removes contaminants and may modify implant surface properties to enhance re-osseointegration potential [50].
Fundamental Mechanisms of Action
The disinfection efficacy of electrolytic systems stems from multiple synergistic mechanisms that can be categorized into three primary modes of action: direct oxidation, indirect oxidation, and physical field effects. Understanding these mechanisms is essential for optimizing decontamination protocols and troubleshooting operational issues.
Direct Oxidation Mechanisms
Direct oxidation occurs when microorganisms come into physical contact with the anode surface within an electrochemical system. This contact triggers irreversible damage to cellular components through several pathways:
- Electron Transfer Reactions: Pathogens adhering to the anode surface experience direct electron withdrawal from their cellular membranes, leading to oxidative destruction of vital cellular structures [48].
- Membrane Disruption: The anode surface generates electrochemical reactions that compromise the structural integrity of bacterial cell walls and membranes, causing irreversible cellular damage [48].
- Intracellular Content Leakage: Following membrane disruption, essential cellular components leak from the compromised pathogens, resulting in rapid cell death and effective microbial inactivation.
The efficiency of direct oxidation is heavily dependent on electrode material selection, electrical parameters, and the duration of exposure, requiring careful optimization for specific applications.
Indirect Oxidation Mechanisms
Indirect oxidation represents a more versatile approach where the electrochemical system generates potent disinfecting agents that diffuse into the solution to inactivate microorganisms. This process involves two primary classes of reactive species:
- Reactive Oxygen Species (ROS): The electrochemical system produces highly reactive compounds including hydrogen peroxide (H₂O₂), ozone (O₃), and hydroxyl radicals (•OH) through water electrolysis. These compounds aggressively oxidize organic materials in bacterial cells, disrupting metabolic functions and causing rapid cell death [48] [49].
- Reactive Chlorine Species (RCS): When chloride ions are present in the solution, the system generates chlorine (Cl₂), hypochlorous acid (HClO), and hypochlorite ions (ClO⁻). These chlorinated oxidants effectively penetrate microbial cells and destroy essential enzymatic systems and genetic material [48] [49].
The following diagram illustrates the sequential electrochemical reactions that produce these disinfecting species:
Electric Field Effects
Beyond chemical oxidation, electrolytic systems generate physical electric fields that contribute to microbial inactivation:
- Electroporation: Applied electric fields create transient pores in microbial cell membranes, facilitating the entry of toxic substances and causing cellular damage [48].
- Membrane Potential Disruption: The natural transmembrane potential of microorganisms is disturbed by external electric fields, interfering with essential cellular processes like ATP production and nutrient transport [48].
These combined mechanisms provide a multi-faceted approach to microbial decontamination that is effective against a broad spectrum of pathogens, including bacteria, viruses, and fungi, making electrolytic technology particularly valuable for complex clinical applications like peri-implantitis treatment.
Electrolytic Cleaning in Dental Implant Research
The application of electrolytic decontamination in dentistry represents a paradigm shift in managing peri-implant diseases. As a novel approach to decontaminating dental implants, electrolytic cleaning effectively removes bacterial biofilm and contaminants without altering surface microtopography or affecting physical properties [50]. This preservation of implant characteristics is crucial for facilitating re-osseointegration following decontamination.
Technology and Implementation
The GalvoSurge system currently stands as the only commercially available device employing electrolytic cleaning for dental implants. This system utilizes sodium formate as an electrolytic solution and applies a maximum current of 600 mA to titanium implants. During the cleaning process, an electrolyte solution is pumped through a platinized ring, generating reactions that remove carbon atoms and convert implant surfaces from hydrophobic to hydrophilic states [50]. This surface transformation enhances bioactivity, promoting better attachment of bone cells and creating favorable conditions for re-osseointegration of previously contaminated implants [50].
Clinical research indicates that electrolytic cleaning, when combined with surgical regenerative procedures, can lead to successful re-osseointegration. Histological evidence from multiple studies confirms that electrolytic decontamination supports new bone formation directly on previously contaminated implant surfaces [50]. However, clinical outcomes vary, with some studies reporting disease resolution while others note recurrence of peri-implantitis, highlighting the need for standardized protocols and further investigation [50].
Comparative Treatment Efficacy
Table 1: Clinical Outcomes of Electrolytic Cleaning for Peri-Implantitis
| Study | Study Design | Implants | Evaluation Period | Re-osseointegration | Disease Resolution |
|---|---|---|---|---|---|
| Gianfreda et al., 2022 | Case Report | 1 | 2 years | Observed | Reported |
| Bosshardt et al., 2022 | Case Report | 4 | 6-13 months | Observed | Recurrence |
| Schlee et al., 2019 | Randomized Controlled Trial | 24 | 6 months | Observed | Recurrence |
| Additional RCT | Randomized Controlled Trial | 24 | 18 months | Observed | Recurrence |
Source: Adapted from [50]
The variability in treatment outcomes highlighted in Table 1 underscores the importance of case selection, surgical technique, and postoperative maintenance in determining clinical success. Electrolytic cleaning appears most effective when implemented as part of a comprehensive treatment protocol that includes mechanical debridement, antimicrobial therapy, and proper regenerative procedures.
Technical Support Center: Troubleshooting Guides and FAQs
Frequently Asked Questions
Q: What is the primary mechanism through which electrolytic cleaning enhances re-osseointegration of dental implants?
A: Electrolytic cleaning facilitates re-osseointegration through two primary mechanisms: first, it thoroughly decontaminates the implant surface by eliminating bacterial biofilm through direct and indirect oxidation processes; second, it modifies the surface characteristics from hydrophobic to hydrophilic, which enhances blood clot stabilization and promotes angiogenesis during healing [50]. This surface transformation creates a more favorable environment for bone cell attachment and new bone formation on previously contaminated implant surfaces.
Q: Why are alkaline solutions typically preferred over acidic solutions in electrolytic cleaning systems?
A: Alkaline solutions are generally preferred because they effectively dissolve greases and oils through saponification, which acids cannot accomplish [51]. Additionally, alkaline solutions reduce the risk of depositing dissolved metallic species onto the component being cleaned, which can occur during cathodic cleaning in acidic environments [51]. Alkaline cleaners also minimize the potential for surface etching or damage that might occur with acidic formulations, particularly on sensitive substrates.
Q: What factors explain the variable clinical outcomes of electrolytic cleaning in treating peri-implantitis?
A: The variability in clinical outcomes stems from multiple factors, including differences in surgical protocols, the severity of bone defects, patient-specific risk factors (such as history of periodontitis or smoking), implant surface characteristics, and adherence to postoperative maintenance programs [33] [50]. The limited and heterogeneous nature of current studies also contributes to apparent outcome variations, highlighting the need for standardized clinical guidelines [50].
Q: Can a pH-neutral solution be effective for electrolytic cleaning, and what are its applications?
A: Yes, pH-neutral solutions like sodium sulfate can be effective for electrolytic cleaning when conduction is established [51]. These solutions provide the benefit of mechanical scrubbing action from gas bubble formation without the chemical aggressiveness of alkaline or acidic electrolytes. This approach is particularly valuable for cleaning sensitive materials that could be damaged by extreme pH conditions, such as certain ceramics or composite surfaces [51].
Q: What are the main challenges associated with implementing electrolytic disinfection systems?
A: Key challenges include managing electrolyte concentration and composition, addressing electrode wear and maintenance requirements, ensuring consistent electrical power availability, and optimizing system parameters for specific applications [49]. Additionally, in dental applications, controlling the procedure to ensure complete decontamination of complex implant geometries while preserving surface characteristics represents a significant technical challenge [50].
Troubleshooting Common Experimental Issues
Problem: Inconsistent Decontamination Results Across Implant Samples
Solution: Ensure consistent electrolyte concentration and composition between experimental runs. Verify that implant surfaces are fully immersed and positioned to allow uniform solution flow across all surfaces. Maintain consistent current application (typically up to 600 mA for dental implants) and treatment duration [50]. Standardize the preoperative contamination protocol to create more consistent baseline conditions across test samples.
Problem: Electrode Degradation and Scaling
Solution: Implement regular electrode maintenance and cleaning protocols. Use anodically inert electrodes such as graphite or specialized coatings to minimize contamination from electrode material [51]. For systems using platinum electrodes, ensure proper polarity and avoid extreme current densities that accelerate wear. Establish a scheduled electrode replacement program based on documented operational hours.
Problem: Inadequate Wetting of Hydrophobic Implant Surfaces
Solution: Pre-treat highly hydrophobic surfaces with a wetting agent to ensure uniform electrolyte contact. Consider incorporating surfactants specifically compatible with electrolytic processes into the electrolyte formulation. Verify that the electrolytic cleaning parameters are sufficient to convert hydrophobic surfaces to hydrophilic states, which is a documented outcome of proper electrolytic cleaning [50].
Problem: Variable Clinical Outcomes in Animal or Human Studies
Solution: Standardize surgical protocols including flap design, degranulation technique, and adjunctive therapies. Implement strict inclusion criteria to control for known risk factors such as history of periodontitis and smoking [33]. Ensure consistent postoperative care and maintenance protocols across all study subjects. Use standardized assessment methods including probing depth, bleeding on probing, and radiographic bone level measurements at consistent time points [50].
Research Reagent Solutions and Experimental Materials
Table 2: Essential Research Materials for Electrolytic Decontamination Studies
| Material/Reagent | Specification | Research Function | Application Notes |
|---|---|---|---|
| Sodium Formate Solution | Electrolyte grade, specific concentration | Primary electrolyte for implant cleaning | Used in GalvoSurge system; removes carbon atoms from implant surfaces [50] |
| Titanium Electrodes | Medical grade, specific dimensions | Anode material for electrolytic systems | Biocompatible; suitable for dental implant research [50] |
| Platinized Ring Electrode | Platinum-coated, specific diameter | Cathode component in commercial systems | Facilitates efficient electron transfer in specialized devices [50] |
| Graphite Electrodes | High purity, various configurations | Alternative inert anode material | Prevents metallic contamination during cleaning processes [51] |
| Sodium Sulfate | Analytical grade, electrolyte | pH-neutral electrolyte solution | Suitable for cleaning sensitive materials without pH extremes [51] |
| Alkaline Cleaning Solutions | Standardized formulations | Removal of organic soils | Effective for saponifying oils and greases from metal surfaces [51] |
| Culture Media for Microbial Assessment | Sterile, specific formulations | Evaluation of decontamination efficacy | Validates microbial reduction post-treatment; assesses biofilm elimination |
Experimental Protocols and Methodologies
Standardized Electrolytic Cleaning Protocol for Dental Implants
Based on analyzed clinical studies, the following protocol outlines a standardized approach for electrolytic decontamination of dental implants in research settings:
Preoperative Assessment: Document clinical parameters including probing depth (6 points per implant: mesial, mesiobuccal, buccal, distobuccal, distal, distolingual), bleeding on probing, and radiographic bone level [50].
Surgical Access: Administer local anesthesia and create full-thickness flaps to expose the defect area. Thoroughly degranulate the peri-implant defect to remove granulomatous tissue [50].
Implant Surface Exposure: Remove the implant suprastructure and place a cover screw. Isolate the surgical field to prevent electrolyte leakage [50].
Electrolytic Cleaning Procedure:
- Apply the electrode ring to encircle the exposed implant surface
- Circulate sodium formate electrolyte solution through the system
- Apply a maximum current of 600 mA for the prescribed duration
- Maintain continuous solution flow across all contaminated surfaces [50]
Adjunctive Therapies: Following decontamination, consider applying adjunctive antimicrobial agents (e.g., rifampicin) or regenerative approaches (guided bone regeneration with platelet aggregates) based on experimental design [50].
Closure and Postoperative Care: Close flaps with appropriate suturing technique. Implement standard postoperative analgesia and instructions [50].
Assessment Timeline: Conduct follow-up assessments at minimum intervals of 6 months, with longer-term evaluation at 12-18 months to monitor re-osseointegration and potential disease recurrence [50].
Outcome Measurement Methodology
To ensure consistent data collection across studies, implement the following standardized measurement protocols:
- Clinical Parameters: Record probing depth (6 points per implant), bleeding on probing (6 points), and plaque index at each assessment time point [50].
- Radiographic Evaluation: Utilize standardized periapical radiographs or CBCT imaging to assess bone level changes and bone fill in defects [50].
- Histological Analysis (in animal studies): Process explanted specimens for histomorphometric analysis to quantify bone-to-implant contact percentage and new bone formation within threads [50].
- Microbiological Assessment: Collect microbial samples from implant surfaces prior to and following decontamination, using standardized culture techniques or molecular methods to quantify bacterial reduction.
The following workflow diagram illustrates the complete experimental process from initial diagnosis through outcome assessment:
Clinical Protocol Implementation and Personalized Risk Management
Troubleshooting Guide: Peri-Implant Maintenance
This guide addresses common challenges in implementing structured supportive care programs for dental implants, providing evidence-based solutions for researchers and clinicians.
Inconsistent Recall Intervals
Problem: Variable recall intervals compromise data collection and patient outcomes in clinical studies. Solution: Implement risk-stratified recall scheduling based on objective criteria [52] [53]:
- Low-risk patients: 6-12 month intervals
- High-risk patients: 3-month intervals (smokers, periodontitis history, poorly controlled diabetes) Validation: Document stability across 2-3 maintenance visits before extending intervals [54].
Unreliable Peri-Implant Probing Data
Problem: Patient discomfort leads to unreliable probing depth measurements. Solution: Use mild infiltration anesthesia (minimal anesthetic) to enable precise, pain-free probing [54]. Protocol: Apply 0.2-0.3N probing force [52] and document baseline measurements at restoration placement [54].
Undetected Early Bone Loss
Problem: Standard radiographs fail to detect subtle marginal bone changes. Solution: Use standardized radiographs with individual positioning stents [54]. Methodology: Create silicone bite registration during baseline radiography and reuse for all follow-ups to ensure identical projection geometry [54].
Implant Surface Damage During Maintenance
Problem: Professional cleaning damages implant surfaces, creating plaque-retentive areas. Solution: Use plastic or titanium instruments for debridement. Avoid abrasive materials that alter surface topography [52]. For research protocols, document instrument composition and force applied.
Floss-Induced Peri-Implant Inflammation
Problem: "Superfloss" fibers detach and cause severe mucosal inflammation within days [54]. Solution: In research protocols, carefully document floss type and inspect for fiber remnants. Consider alternative interdental cleaning methods where appropriate.
Table 1: Evidence-Based Recall Interval Recommendations
| Risk Category | Recall Interval | Key Monitoring Parameters | Supporting Evidence |
|---|---|---|---|
| Low risk (no risk factors, good hygiene) | 12 months | BOP, plaque scores, patient compliance | [53] |
| Moderate risk (1-2 risk factors) | 6 months | Probing depths, radiographic bone levels | [52] [53] |
| High risk (periodontitis history, smoker, poor hygiene) | 3 months | All clinical parameters + radiographic assessment | [54] [52] |
| Post-treatment (after peri-implantitis therapy) | 3 months | BOP, suppuration, probing depth changes | [55] |
Monitoring Protocol Specifications
Clinical Parameter Documentation
Table 2: Essential Peri-Implant Monitoring Parameters
| Parameter | Measurement Method | Frequency | Normal Range | Critical Values |
|---|---|---|---|---|
| Plaque Index | Mombelli modified plaque index (mPI) [52] | Every recall | Score 0-1 | Score ≥2 |
| Bleeding on Probing (BOP) | Apse et al. modified gingival index [52] | Every recall | Score 0-1 | Score ≥2 |
| Probing Depth | Light force (0.2-0.3N), 4-6 sites per implant [52] | Every recall | ≤4mm | ≥5mm or increasing |
| Suppuration | Digital pressure from apical to coronal [54] | Every recall | Absent | Present |
| Radiographic bone level | Long-cone paralleling technique with stent [54] [52] | Annually (high risk) or when pathology suspected | <1.5mm first year, <0.2mm/year thereafter | Progressive bone loss |
Experimental Protocol: Ten-Second Technique for Surface Decontamination
Background: Novel protocol for managing peri-implant disease in research settings [29].
Materials:
- Hybenx gel (EPIEN Medical, USA)
- Air-polishing device (PROPHYflex 4) with 40μm sodium bicarbonate powder
- Ultrasonic device with thin inserts (PIEZON PS Perio Slim)
- Scanning electron microscope for validation
Methodology:
- Macroscopic debridement: Use ultrasonic ablation with thin inserts to remove gross debris
- Chemical application: Apply Hybenx gel to contaminated implant surfaces for 10 seconds
- Mechanical cleansing: Use air-polishing device with sodium bicarbonate powder water spray
- Rinsing: Rinse for 30 seconds with air-polishing device water spray
- Aspiration: Immediately aspirate effervescent foam (water, CO₂, Na₂SO₄)
- Final cleansing: Continue water spray for 1-2 minutes until visually clean surface achieved
Validation: Assess surface decontamination using SEM and EDX analysis [29].
Frequently Asked Questions
Q: What is the minimum evidence-based recall interval for all implant patients? A: Annual recall represents the minimum frequency for even low-risk patients, as some monitoring is essential for all implant cases [53].
Q: How can researchers standardize radiographic bone level assessment? A: Use individual positioning stents created at baseline examination with quick-setting A-silicone material to ensure identical projection geometry across all follow-up visits [54].
Q: What bleeding pattern indicates peri-implant health versus disease? A: Healthy sites show 0% BOP, peri-implant mucositis sites show ~67% BOP, and peri-implantitis sites show ~91% BOP [52].
Q: Which oral hygiene tools risk damaging implant surfaces? A: Conventional metal scalers can alter implant surface topography. Use plastic, titanium, or gold-plated instruments instead [52].
Q: What is the clinical significance of suppuration detection? A: Suppuration indicates active peri-implant disease requiring intervention. Detect by drying the sulcus and applying light apical-to-coronal pressure with a fingertip [54].
Research Reagent Solutions
Table 3: Essential Reagents for Peri-Implant Maintenance Research
| Reagent/Equipment | Research Application | Function | Protocol Specifications |
|---|---|---|---|
| Hybenx gel | Surface decontamination studies | Chemical debridement via Desiccation Shock Debridement technology | 10-second application time [29] |
| Sodium bicarbonate powder (40μm) | Air-polishing research | Mechanical biofilm removal | Used with air-polishing devices [29] |
| Chlorhexidine digluconate (0.12-0.2%) | Mucositis management studies | Antimicrobial adjunct | Mouthwash or local gel application [56] |
| Individual radiographic stents | Standardized bone loss assessment | Ensure identical projection geometry | Create with A-silicone during baseline radiography [54] |
| Plastic/Titanium scalers | Implant surface integrity studies | Plaque removal without surface damage | Alternative to metal instruments [52] |
Experimental Workflows
Maintenance Protocol Decision Pathway
Surface Decontamination Experimental Workflow
Peri-implantitis, an inflammatory condition affecting tissues around dental implants, leads to progressive bone loss and threatens implant longevity. With subject-based prevalence reported between 18.8% and 34%, effective decontamination of implant surfaces has become a critical focus in dental implant research [57]. The complex topography of modern dental implants—featuring undercuts, grooves, and porosities—creates significant challenges for complete biofilm removal, a prerequisite for preventing disease progression and achieving re-osseointegration [58].
This technical support center addresses the key methodological considerations for researchers investigating three primary decontamination modalities: air polishing, ultrasonic scaling, and laser therapy. The protocols and troubleshooting guides below are framed within the context of peri-implantitis prevention research, providing evidence-based methodologies for in vitro and clinical investigations.
Comparative Efficacy Data Tables
Table 1: Clinical Outcomes of Decontamination Techniques for Peri-Implant Mucositis (6-Month Follow-Up)
| Parameter | Er:YAG Laser | Ultrasonic Scaler | Statistical Significance |
|---|---|---|---|
| Full Mouth Bleeding (FMBoP) | 30.1% → 21.5% (p<0.001) | 35.0% → 30.0% (p<0.01) | p < 0.05 at 6 months |
| Full Mouth Plaque (FMPS) | 61.5% → 32.7% (p<0.001) | 58.7% → 39.1% (p<0.001) | Not significant |
| Implant Bleeding on Probing (BoP) | 89.0% → 55.7% (p<0.001) | 94.9% → 63.7% (p<0.001) | Not significant |
| Implant Plaque Score | Not reported | Not reported | p < 0.05 at 6 months |
| PPD ≥ 4 mm (at 3 months) | 43.5% | 73.9% | p < 0.05 |
| Patient Pain (VAS at 3 days) | 0.08 | 0.2 | p < 0.05 |
Data adapted from clinical trial comparing Er:YAG laser to ultrasonic scaler for peri-implant mucositis treatment [59].
Table 2: Non-Surgical Treatment Outcomes for Peri-Implantitis (3-Month Follow-Up)
| Outcome Measure | Erythritol Air Polishing | Ulasonic Therapy (PEEK tip) | Statistical Significance |
|---|---|---|---|
| Bleeding on Probing (BoP) | -0.037 (95% CI: -0.147; 0.073) | Reference | p = 0.380 |
| Suppuration on Probing (SoP) | No significant difference | No significant difference | Not significant |
| Plaque Score (Plq) | No significant difference | No significant difference | Not significant |
| Probing Pocket Depth (PPD) | No significant difference | No significant difference | Not significant |
| Treatment Pain/Discomfort (VAS) | 2.1 (±1.9) | 2.6 (±1.9) | p = 0.222 |
| Disease Resolution Rate | 18.4% (at 3 months) | 18.4% (at 3 months) | Not significant |
Data adapted from randomized controlled trial comparing erythritol air polishing to ultrasonic therapy for peri-implantitis [60].
Experimental Protocols
Protocol 1: Er:YAG Laser Application for Peri-Implant Mucositis
Based on Methodology from: Clinical trial assessing Er:YAG laser for peri-implant mucositis [59]
- Laser Parameters: Er:YAG laser, wavelength 2940 nm
- Application Protocol:
- Treatments performed at baseline, 3 months, and 6 months
- Combine with oral hygiene instruction and plaque registration
- Guide patients in proper cleaning technique using toothbrush and interproximal aids
- Assessment Schedule:
- Record Full Mouth Bleeding on Probing (FMBoP) and Full Mouth Plaque Score (FMPS) at each session
- Assess implant-specific parameters: Bleeding on Probing (BoP), modified Bleeding Index (mBI), Probing Pocket Depth (PPD), suppuration
- Evaluate patient-reported outcomes: Oral Health-Related Quality of Life (OHIP-14) and Visual Analog Scale (VAS) for pain and aesthetics
- Outcome Measures:
- Primary: Reduction in bleeding and plaque scores
- Secondary: Patient-reported pain and satisfaction, radiographic bone levels
Protocol 2: Erythritol Air Polishing for Peri-Implantitis
Based on Methodology from: RCT comparing air polishing to ultrasonic therapy [60]
- Equipment Setup:
- Air polisher with erythritol-based powder (14μm grain size) containing 0.3% chlorhexidine
- Plastic nozzle with trilateral powder-outlet and apical water-only spray
- Device Settings:
- Pressure: Maximum liquid pressure 5.0 bar, air-powder pressure approximately 7 bar (75% of maximum)
- Application time: 30 seconds per implant (5 seconds per implant site)
- Pre-treatment Procedures:
- Check for hard deposits (calculus) and remove with hand instruments if present
- Precede treatment with 30-second antimicrobial mouth rinse
- Use local anesthesia as needed
- Post-treatment Assessment:
- Evaluate at 3 months for treatment success
- Success criteria: PPD <5 mm, no bleeding/suppuration on probing, no progressive bone loss
Protocol 3: Surface Alteration Analysis of Decontamination Methods
Based on Methodology from: In vitro study of implant surface alterations [57]
- Sample Preparation:
- Use titanium disks with moderately rough SLA (sandblasted and acid-etched) surface
- Standardize surface roughness to Sa 1.30 μm
- Sterilize by autoclaving before procedures
- Test Conditions:
- Air powder abrasion (AP) with glycine powder, 40s application, 90° angle, 2mm distance
- Titanium brush (TB) at 600 rpm for 40s under continuous irrigation
- Diode laser (810nm) at 3W and 4W power, 40s application, minimal non-contact distance
- Analysis Methods:
- SEM Analysis: Field emission gun SEM at different magnifications with 10 kV potential difference
- Topographical Analysis: White light interferometer with three 0.71 × 0.53 mm areas per sample
- Roughness Parameters: Sa (arithmetic average roughness), Sq (average quadratic roughness), Scx (summit spacing)
- Chemical Analysis: X-ray photoelectron spectroscopy (XPS) for surface chemical composition
Troubleshooting Guides & FAQs
Frequently Asked Technical Questions
Q: What decontamination method causes the least surface alteration to titanium implants? A: Based on in vitro analysis, air powder abrasion with glycine powder and diode laser at 3W power showed the lowest impact on surface morphology and chemical composition. Titanium brushes caused significant alterations including coarse scratches and smoothed titanium portions, while higher power laser (4W) showed signs of localized melting due to temperature rise [57].
Q: How do air polishing powders compare in terms of surface damage to restorations? A: Systematic review data indicates air polishing with erythritol and glycine powders causes significantly less surface roughness compared to sodium bicarbonate and calcium carbonate powders. Surface roughness for resin-based composites (RBCs) and resin-modified glass ionomer cements (RMGICs) were most affected by ultrasonic instrumentation, while zirconia (ZrO2) and lithium disilicate (LDS) showed highest resistance to surface changes [61].
Q: What is the recommended protocol for managing subcutaneous emphysema from air polishing? A: Although rare, subcutaneous emphysema requires immediate intervention [62]:
- Immediately stop the procedure
- Perform differential diagnosis to distinguish from allergic reaction or infection
- Palpate affected area to determine extent of emphysema
- Monitor for airway compromise, cardiac function, vision changes
- Consider prophylactic antibiotics and/or corticosteroids
- Refer for specialist care (otolaryngology) if significant symptoms present
Q: Which mechanical decontamination method shows superior clinical outcomes for peri-implantitis? A: Current evidence suggests no statistically significant difference in clinical outcomes between erythritol air polishing and ultrasonic scaling at 3-month follow-up for parameters including BoP, SoP, plaque scores, PPD, and patient discomfort. Both methods showed limited disease resolution (18.4%), indicating most patients required further surgical treatment [60].
Common Experimental Challenges
Challenge: Inconsistent Decontamination Across Implant Surface Topographies
- Solution: Implement multiple analysis points (recommended: 4 sides per implant) rotated at 90° intervals with both SEM and EDX measurements to ensure comprehensive surface characterization [63].
Challenge: Controlling for Operator-Induced Variables in Decontamination Studies
- Solution: Use standardized application times (e.g., 30s per implant, 5s per site) and predefined device settings as outlined in manufacturer guidelines. Implement blinded examiners for outcome assessment to minimize measurement bias [59] [60].
Challenge: Assessing Biofilm Removal Efficacy Without Altering Implant Surface
- Solution: Employ SEM-EDX analysis protocol that does not require sputtering or metallization of samples before observation. Use secondary electron images at standardized magnifications to evaluate residual biofilm and surface changes simultaneously [63].
The Scientist's Toolkit
Table 3: Essential Research Reagents and Materials
| Item | Specification/Function | Research Application |
|---|---|---|
| Er:YAG Laser | 2940 nm wavelength, variable energy settings | Bacterial reduction and surface decontamination |
| Erythritol Powder | 14μm grain size, often with 0.3% CHX additive | Air polishing with reduced surface alteration |
| Glycine Powder | Fine powder, minimal abrasiveness | Alternative to erythritol for air polishing |
| Titanium Brushes | 600 rpm rotation under irrigation | Mechanical biofilm removal |
| PEEK Ultrasonic Tips | Plastic-coated tips for implant surfaces | Ultrasonic scaling without surface damage |
| Hybenx Gel | Sulfonated phenolics, sulfuric acid, water | Chemical decontamination via Desiccation Shock Debridement |
| Sodium Bicarbonate Powder | 40μm particles for air polishing | Biofilm removal in Ten-Second Technique protocol |
| Diode Laser | 810nm wavelength, 3-4W power settings | Alternative laser decontamination method |
Methodological Workflows
Diagram 1: Decontamination Technique Selection Algorithm
Diagram 2: Experimental Workflow for Surface Analysis Study
Key Technical Recommendations
Based on current evidence, researchers should consider the following methodological approaches:
For clinical efficacy studies: Implement a randomized controlled trial design with blinded examiners, standardized application times, and multiple follow-up points (minimum 3 and 6 months) to assess both clinical parameters and patient-reported outcomes [59] [60].
For surface analysis studies: Utilize multiple complementary analysis methods (SEM, white light interferometry, XPS) to comprehensively evaluate both morphological and chemical surface alterations following decontamination protocols [57].
For biofilm removal studies: Consider the Ten-Second Technique protocol combining Hybenx gel with subsequent air polishing for enhanced decontamination, particularly for surgical applications [63].
For safety and adverse event monitoring: Include specific protocols for managing rare complications such as subcutaneous emphysema, particularly when studying air polishing techniques in compromised patients [62].
These optimized decontamination protocols and troubleshooting guidelines provide a methodological framework for advancing research in peri-implantitis prevention and treatment, with the ultimate goal of improving long-term implant success rates.
Troubleshooting Guides
Patient Risk Stratification and Pre-Operative Planning
Q: What are the primary risk factors for peri-implantitis I should screen for during patient selection?
A: Evidence consistently identifies several key risk factors that significantly increase the probability of biological complications. A 2024 consensus report from the Academy of Osseointegration and American Academy of Periodontology (AO/AAP) and a 2025 retrospective analysis provide a clear hierarchy of major risks [55] [64].
Table: Key Risk Factors for Peri-Implantitis
| Risk Category | Specific Factor | Clinical Implications |
|---|---|---|
| Systemic & Behavioral | History of Periodontitis | Strongest risk indicator; requires periodontal stabilization prior to implant placement [55]. |
| Smoking | Impairs healing and immune response; dose-dependent effect [55] [64]. | |
| Uncontrolled Diabetes | Compromises wound healing and increases susceptibility to infection [55] [64]. | |
| Poor Oral Hygiene | Directly leads to biofilm accumulation, triggering inflammation [55]. | |
| Local & Procedural | Implant Malposition | Creates areas difficult to clean and compromises prosthetic emergence profile [55]. |
| Unfavorable Prosthetic Design | Over-contouring, poor embrasure spaces hinder maintenance [55] [64]. | |
| Suboptimal Soft Tissue Phenotype | Thin biotype more prone to recession and inflammation [55]. |
Q: How does a history of periodontitis specifically alter the risk profile for a patient receiving implants?
A: Patients with a history of periodontitis are considered the highest risk group for developing peri-implantitis. The condition indicates a susceptible host response to bacterial biofilm, which can similarly affect the tissues surrounding implants [55]. The risk is not just bacterial transmission but also a shared genetic and immunological predisposition for an exaggerated inflammatory response. Meticulous pre-operative therapy to achieve periodontal health and a strict, lifelong supportive maintenance schedule are non-negotiable for these patients [65].
Intra-Operative and Prosthetic Management
Q: What are the most critical surgical and prosthetic errors that predispose implants to failure in high-risk patients?
A: Technical execution is paramount, especially when biological resilience is already compromised. Common errors include [65]:
- Poor Implant Positioning: Placing an implant outside the prosthetic envelope or too close to an adjacent tooth creates uncleanable areas and biomechanical strain, leading to bone loss [55].
- Inadequate Flap Design/Closure: In regenerative procedures, excessive flap tension or poor passive closure can lead to membrane exposure, infection, and graft failure [65].
- Prosthetic Over-contouring: Bulky crown and abutment contours impede the patient's and clinician's ability to perform effective plaque control, directly promoting peri-implant mucositis, which can progress to peri-implantitis [55] [64].
Logical flow from clinical errors to implant failure
Post-Operative and Maintenance Management
Q: What is the most effective non-surgical protocol for managing peri-implant mucositis in a systemically compromised patient (e.g., a controlled diabetic)?
A: Peri-implant mucositis is reversible with effective biofilm disruption. The foundation of treatment is Mechanical Debridement (MD). However, for high-risk patients, evidence supports the use of adjunctive therapies to enhance antimicrobial efficacy. A 2025 systematic review and meta-analysis found that combining MD with Photodynamic Therapy (PDT) led to significantly greater reductions in probing pocket depth (PPD) and bleeding on probing (BoP) compared to MD alone at 3- and 6-month follow-ups [66].
Table: Quantitative Outcomes of Adjunctive PDT vs. MD Alone
| Outcome Measure | Condition | Intervention | Follow-up | Mean Difference (mm or %) | 95% CI |
|---|---|---|---|---|---|
| PPD Reduction | Peri-implant Mucositis | MD + PDT | 3 months | -0.95 mm | [-1.76 to -0.14] |
| PPD Reduction | Peri-implantitis | MD + PDT | 3 months | -0.86 mm | [-1.21 to -0.51] |
| PPD Reduction | Peri-implantitis | MD + PDT | 6 months | -0.83 mm | [-1.62 to -0.04] |
| BoP Reduction | Peri-implantitis | MD + PDT | 6 months | -6.76 % | N/A |
Data adapted from [66]. MD: Mechanical Debridement; PDT: Photodynamic Therapy.
Experimental Protocol: Adjunctive Photodynamic Therapy (PDT) for Peri-Implant Mucositis
- Mechanical Debridement: Thoroughly debride the implant surface using titanium or plastic curettes and an ultrasonic/piezoelectric device with a plastic tip to remove supra- and subgingival biofilm and calculus.
- Application of Photosensitizer: Isolate the implant site and dry the peri-implant pocket. Apply a photosensitizing agent (e.g., Toluidine Blue O or Methylene Blue) into the pocket using a blunt cannula or micro-brush. Allow it to incubate for the recommended time (typically 60 seconds) to bind to the remaining bacteria.
- Laser Activation: Insert a low-level laser fiber optic tip into the pocket. Activate the laser at the specified wavelength (e.g., 660 nm for diode lasers) to activate the photosensitizer. The recommended energy density is typically 0.5-1.0 J/cm² per site.
- Irrigation and Re-assessment: Thoroughly irrigate the site with saline to remove the photosensitizer and debris. Re-assess clinical parameters (BoP, PPD) and schedule the patient for supportive peri-implant maintenance within 3 months [66].
Frequently Asked Questions (FAQs)
Q: Are there any emerging implant surface technologies designed to prevent peri-implantitis in high-risk individuals?
A: Yes, the development of antimicrobial coatings is a major research focus. These strategies are classified based on their goal: prevention versus treatment. Preventive coatings are designed to be durable and withstand the oral environment indefinitely, often by creating a surface that resists biofilm formation. Therapeutic coatings, used when disease is established, are "smart" and responsive to stimuli (e.g., pH changes in an inflamed environment) to release antimicrobial agents on demand [67]. While promising, most are still in experimental stages, and no coating has achieved widespread commercial success for this indication yet [67].
Q: What are the predominant causes of late dental implant failure, and how can they be mitigated?
A: Late failures (after osseointegration) are predominantly associated with peri-implantitis and biomechanical overload [64] [68]. Underlying causes often include a history of periodontitis, poor plaque control, and the absence of regular maintenance care [64]. Mitigation requires a comprehensive approach: rigorous patient selection, impeccable prosthetic design to distribute forces evenly, and a lifelong commitment to supportive peri-implant therapy with regular monitoring of bone levels and soft tissue health [55] [65].
Q: If an implant fails, what are the available retrieval techniques?
A: The choice of technique depends on the reason for failure and the amount of remaining bone. Common methods include [68]:
- Trephine Burs: The most frequently reported method; used to remove the implant and a small ring of surrounding bone. Success rates for atraumatic retrieval are high (70-100%), but it is the most invasive bone-removing technique.
- Reverse-Torque Devices: Used to unscrew integrated implants, suitable for explained implants without severe complications.
- Ultrasonic/Piezoelectric Methods: Minimally invasive techniques that use high-frequency vibrations to section and remove the implant with minimal damage to surrounding bone.
- Laser-Assisted Removal: An emerging technique investigated for its precision.
The Scientist's Toolkit: Research Reagent Solutions
Table: Essential Materials for Investigating Peri-Implantitis
| Reagent / Material | Function in Experimental Model |
|---|---|
| Titanium & Plastic Curettes | Standard mechanical debridement control in surface decontamination studies [55] [66]. |
| Photosensitizers (e.g., Toluidine Blue O) | Key reagent for Photodynamic Therapy (PDT); binds to bacterial biofilms for targeted destruction upon laser activation [66]. |
| Low-Level Laser Systems (660 nm Diode) | Activates photosensitizers in PDT protocols to generate reactive oxygen species that are bactericidal [66]. |
| Antimicrobial Coating Formulations | Experimental surfaces (e.g., chitosan, silver nanoparticles) tested for their ability to resist biofilm formation or provide on-demand drug release [67]. |
| Selective Culture Media / PCR Assays | For identifying and quantifying peri-implant pathogens (e.g., Fusobacterium, Bacteroides, Treponema species) in microbiological studies [66]. |
Research strategies for peri-implantitis prevention
Prosthetic Design Considerations to Facilitate Maintenance and Prevent Disease
Troubleshooting Guide: Common Prosthetic-Related Complications
How does prosthetic design contribute to food entrapment and peri-implant disease?
Food entrapment around implant restorations is frequently traced to an inadequate emergence profile—the transition between the implant platform and the final crown. A poorly designed profile creates inaccessible areas for hygiene, leading to plaque retention, inflammation, and peri-implant mucositis, a precursor to peri-implantitis [69].
- Root Cause: Often due to poor crown contouring, lack of papilla support, misaligned implant position, or the use of generic stock abutments that fail to match the individual's gingival architecture [69].
- Solution: Design an ideal emergence profile that supports soft tissue and mimics natural tooth contours. This requires considering tissue thickness and crown emergence angle, and aiming for smooth, convex profiles to reduce plaque traps. Custom abutments are highly recommended for precise tailoring to the patient's tissue and prosthetic needs, especially when implant angle or depth is suboptimal [69].
What is the association between the emergence profile and bone loss?
Overcontouring of the prosthesis emergence profile and an excessive mucosal emergence angle are strongly associated with marginal bone loss, soft tissue recession, and peri-implantitis [70]. An abrupt or convex transition from the implant platform can prevent proper self-performed hygiene and disrupt the biological seal.
- Preventive Strategy: Develop prosthetic contours with a subcritical and critical design. The subcritical zone (within the sulcus) should provide space for soft tissues to achieve a good seal, while the critical zone (at the gingival margin) should be convex to provide necessary tissue support [71].
Why is there a grey hue at the gingival margin, and how can it be resolved?
A dark or grey appearance at the gumline is an aesthetic complication that occurs when a thin gingival biotype allows the metallic color of a titanium abutment or implant collar to show through [69].
- Primary Causes: Thin soft tissue biotype, shallow implant placement, or labial implant angulation [69].
- Corrective and Preventive Actions:
What are the consequences of a malpositioned dental implant on prosthetic maintenance?
Malpositioned implants (incorrect angulation or depth) represent a significant restorative challenge, directly impacting the feasibility of creating a maintainable prosthesis [69].
- Risks: Compromised aesthetics, food traps, difficult hygiene access, and biomechanical overload due to non-axial force distribution [69].
- Restorative Workarounds: Solutions include angled abutments, custom abutments with milled screw access channels, and mesio-structures. However, these can introduce new issues like prosthetic bulk or reduced cleansability [69].
- Ultimate Prevention: Utilize guided surgery and digital treatment planning to align the final prosthetic plan with surgical execution, ensuring optimal 3D implant position [69] [71].
Screw-retained vs. Cement-retained: Which prosthetic design is better for maintenance?
The choice of retention impacts retrievability and the risk of biological complications.
- Screw-Retained Restorations: Considered the gold standard for maintainability, as they allow the prosthesis to be removed for professional monitoring, cleaning, and treatment of peri-implant diseases [71].
- Cement-Retained Restorations: Pose a risk of excess cement remnants, which are challenging to detect and remove and are a known etiological factor for peri-implantitis [71]. If used, meticulous cementation technique is critical.
- Hybrid Alternative (Screw-Cement Retained): This technique involves cementing a crown onto a Ti-base abutment extra-orally. It combines the biomechanical advantages of cementation with the retrievability of a screwed prosthesis, as the crown can be unscrewed from the implant [71].
Frequently Asked Questions (FAQs) for Researchers
FAQ 1: What are the key prosthetic-related risk factors for peri-implantitis identified in recent consensus reports?
The 2024 Academy of Osseointegration/American Academy of Periodontology (AO/AAP) consensus identifies several local prosthetic factors as key risk indicators [55]. These include:
- Implant malposition
- Unfavorable prosthetic design that impedes oral hygiene
- Suboptimal peri-implant soft tissue phenotypical features (e.g., thin tissue biotype)
The consensus emphasizes that prevention and management must address these site-related factors through tailored prosthetic designs and ongoing supportive care [55].
FAQ 2: How do different abutment materials influence the peri-implant environment?
Abutment material selection directly affects bacterial adhesion and soft tissue attachment, which are critical for maintaining a healthy biological seal.
- Zirconia Abutments: Demonstrate less bacterial adhesion compared to titanium in vitro. They are also superior for aesthetics, preventing grey show-through in thin biotypes [71].
- Titanium Abutments: A longstanding, reliable choice, but their metallic color can be a disadvantage in aesthetic zones. Surface characteristics and microgeometry can influence plaque retention [70] [69].
FAQ 3: What is the role of multi-unit abutments in complex prosthetic cases?
For multiple implants, especially those with significant divergence in axes, multi-unit abutments offer several biological and mechanical benefits [71]:
- Passive Fit: They make it easier to achieve a passive fit for the prosthesis, reducing mechanical stress.
- Biological Transformation: When used on a bone-level implant, they effectively convert it to a tissue-level design. A clinical trial demonstrated that using multi-units with a height of more than 2mm resulted in almost no initial bone loss [71].
- Tissue Preservation: They minimize the need for repeated disconnections and reconnections of components at the bone level, which can disrupt the fragile hemidesmosomal attachment and lead to bone resorption [71].
Experimental Protocols for Prosthetic Research
Protocol 1: In-Vitro Assessment of Implant Surface Decontamination
This protocol is adapted from a 2025 in vitro study exploring the "Ten Second Technique" (TST) for managing peri-implant disease [29].
Aim: To evaluate the efficacy of a novel decontamination method on contaminated implant surfaces.
Materials and Reagents:
- Explanted contaminated implants or in vitro contaminated test implants.
- Hybenex gel (EPIEN Medical, USA).
- Air-polishing device (e.g., PROPHYflex 4) with 40 μm sodium bicarbonate powder (e.g., Airflow Comfort).
- Ultrasonic device with thin inserts (e.g., PIEZON PS Perio Slim).
- Scanning Electron Microscope (SEM) and Energy Dispersive X-ray (EDX) analyzer.
Methodology:
- Macroscopic Debridement: Pretreat implant surfaces with ultrasonic ablation using thin inserts to remove gross debris [29].
- Chemical Application: Apply Hybenex gel to the contaminated implant surfaces for exactly 10 seconds [29].
- Mechanical Air Polishing: Rinse the gel for 30 seconds using an air-polishing device with a sodium bicarbonate powder water spray. This creates an effervescent foam that aids in debris removal [29].
- Final Rinse: Use a 1-2 minute water spray until a visually clean surface is achieved. Aspirate all residues immediately [29].
- Analysis:
- Use SEM to qualitatively assess biofilm removal and inspect for surface alterations at various magnifications.
- Use EDX for semi-quantitative elemental analysis to measure changes in surface contamination (e.g., carbon content from biofilm) before and after TST application. Perform multiple measurements on different implant sides.
- Employ One-Way ANOVA and Tukey's multiple comparison tests to determine statistical significance (p < 0.05) in contamination levels [29].
Protocol 2: Evaluating the Plaque Retention Properties of Prosthetic Materials
Aim: To compare the amount of microbial biofilm adhesion on different abutment and crown materials.
Materials and Reagents:
- Test specimens (e.g., polished titanium, roughened titanium, polished zirconia, monolithic zirconia, PMMA).
- Bacterial strains relevant to peri-implant disease (e.g., Streptococcus oralis, Fusobacterium nucleatum, Porphyromonas gingivalis).
- Culture media (e.g., Brain Heart Infusion broth).
- Staining methods (e.g., Crystal Violet) or fluorescent dyes for biomass quantification.
- Confocal Laser Scanning Microscope (CLSM) or spectrophotometer.
Methodology:
- Specimen Preparation: Fabricate discs or contoured specimens of the test materials, ensuring standardized surface roughness and sterilization.
- Biofilm Formation: Inoculate specimens with a bacterial suspension and incubate under appropriate conditions (e.g., 37°C, anaerobic atmosphere) for a set period (e.g., 24-72 hours) to allow for biofilm formation.
- Biofilm Quantification:
- Crystal Violet Assay: Fix the biofilm, stain with Crystal Violet, elute the dye, and measure absorbance with a spectrophotometer to quantify total attached biomass.
- CLSM Analysis: Use fluorescent dyes (e.g., LIVE/DEAD BacLight) to stain the biofilm. Use CLSM to generate 3D images and quantify biofilm thickness, biovolume, and bacterial viability.
- Statistical Analysis: Compare quantitative data between material groups using appropriate statistical tests (e.g., ANOVA).
Research Reagent Solutions
The following table details key materials and their functions in research related to prosthetic design and peri-implant disease.
| Reagent/Material | Primary Function in Research |
|---|---|
| Hybenex Gel | A chemical decontaminant used in in vitro and clinical studies to eliminate biofilm and molecular debris from contaminated implant surfaces via its Desiccation Shock Debridement (DSD) technology [29]. |
| Sodium Bicarbonate Powder (40μm) | Used with air-polishing devices for mechanical debridement and cleansing of implant surfaces in experimental decontamination protocols [29]. |
| Zirconia Abutment Blanks | Material for fabricating custom abutments; studied for its superior biocompatibility, low plaque adhesion, and favorable soft tissue response compared to titanium [71]. |
| Ti-Base Abutments | The metallic component used in screw-cement retained hybrid prostheses; allows for combination with various crown materials (zirconia, PMMA) while maintaining retrievability [71]. |
| Monolithic Zirconia | A restorative material for definitive prostheses; investigated for its high strength, low fracture incidence, and superior biocompatibility leading to less plaque accumulation, especially in ultra-polished finishes [71]. |
| Reinforced PMMA | A polymer used for definitive prostheses in research settings; studied as a potential alternative to classic materials due to its low weight, biocompatibility, and enhanced mechanical properties [71]. |
Visual Workflows: Prosthetic Design and Experimental Analysis
Diagram 1: Prosthetic Design Logic for Peri-Implant Health
Prosthetic design logic for peri-implant health - This flowchart outlines the key decision points in prosthetic design that influence long-term maintenance and disease prevention. Green pathways represent choices that support health, while red pathways indicate higher-risk options [70] [69] [71].
Diagram 2: Implant Surface Decontamination Workflow
Implant surface decontamination workflow - This diagram visualizes the experimental protocol for the "Ten Second Technique" (TST), a method for decontaminating implant surfaces in peri-implantitis research [29].
Diagnostic and Monitoring Protocols for Peri-Implant Tissues
Routine monitoring using validated diagnostic parameters is essential for detecting early inflammatory changes in peri-implant tissues. The table below summarizes key clinical and radiographic indicators used to assess peri-implant health and disease activity [52].
| Parameter | Assessment Method | Healthy Implant Indicators | Peri-Implant Disease Indicators |
|---|---|---|---|
| Bleeding on Probing (BOP) | Gentle probing (0.2-0.3 N force) with a plastic or titanium probe [52]. | Absence of bleeding [52]. | Presence of bleeding; sites with peri-implantitis show BOP in 91% of cases [52]. |
| Probing Depth (PD) | Measurement with a calibrated periodontal probe [52]. | ~3 mm depth [52]. | Pockets ≥5 mm, which can serve as a protected niche for bacteria [52]. |
| Plaque Index | Visual assessment or running a probe across the implant surface (modified Plaque Index, mPI) [52]. | No detection of plaque (Score 0) [52]. | Visible plaque (Score 2) or abundance of soft matter (Score 3) [52]. |
| Mucosal Inflammation | Visual assessment of color, edema, and glazing (modified Gingival Index, mGI) [52]. | Normal mucosa, no bleeding (Score 0) [52]. | Redness, edema, bleeding forming a confluent red line (Score 2) [52]. |
| Radiographic Bone Level | Long-cone paralleling technique with positioning devices [52]. | Mean bone loss <1.5 mm first year post-loading; <0.2 mm/year thereafter [52]. | Progressive bone loss exceeding success criteria [52]. |
| Suppuration | Visual observation or gentle pressure on the peri-implant sulcus [52]. | No suppuration [52]. | Presence of pus, indicating active infection and need for anti-infective therapy [52]. |
Experimental Protocol: Standardized Peri-Implant Probing
Objective: To consistently measure peri-implant probing depths and assess bleeding on probing to monitor tissue health. Methodology:
- Use a plastic or titanium periodontal probe to prevent scratching the implant surface.
- Apply a controlled, light force of 0.2-0.3 N.
- Probe at six sites per implant (mesio-buccal, mid-buccal, disto-buccal, mesio-lingual, mid-lingual, disto-lingual).
- Record probing depths to the nearest millimeter.
- Note the presence or absence of bleeding within 30 seconds of probing.
- Dip the probe in chlorhexidine prior to use to avoid bacterial infiltration of the sulcus [52].
Evidence-Based Home Care Techniques for Patients
Effective daily plaque control is the cornerstone of preventing peri-implant mucositis and its progression to peri-implantitis. The following table compares the efficacy of various home care tools as established in clinical studies [52] [72] [73].
| Technique | Recommended Tools | Protocol | Mechanism of Action |
|---|---|---|---|
| Brushing | Soft-bristled or electric toothbrush; non-abrasive toothpaste [72] [73] [74]. | Brush twice daily for two minutes, positioning bristles at a 45-degree angle to the gumline using gentle circular motions [73]. | Mechanically disrupts supragingival biofilm without damaging the implant surface or irritating peri-implant tissues [73]. |
| Interdental Cleaning | Implant-specific floss, super floss, floss threaders, interdental brushes (plastic-coated), water flossers [52] [72] [73]. | Clean daily. Thread floss under the implant crown; use brushes and water flossers to clean abutments and the gumline [73]. | Removes biofilm and debris from the implant collar, abutment connections, and interproximal areas that are inaccessible to toothbrushes [73]. |
| Antimicrobial Rinsing | Alcohol-free, antimicrobial mouthwash (e.g., Chlorhexidine-based) [74]. | Rinse daily as an adjunct to mechanical cleaning, following product instructions [74]. | Reduces the overall bacterial load in the oral cavity and helps control plaque biofilm [74]. |
Experimental Protocol: Evaluating Biofilm Removal Efficacy of Home Care Tools
Objective: To compare the biofilm-removal efficacy of different oral hygiene instruments on titanium implant surfaces in an in-vitro model. Methodology:
- Grow a standardized oral biofilm on titanium discs in a bioreactor.
- Divide discs into test groups: soft manual brush, electric toothbrush, interdental brush, water flosser, and control (no cleaning).
- Subject discs to a standardized cleaning cycle simulating human use.
- Quantify residual biofilm using viable plate counts and scanning electron microscopy (SEM).
- Analyze surface topography of titanium discs post-cleaning for any abrasive damage.
Professional Intervention and Supportive Therapy
When home care is insufficient to prevent disease, professional interventions are required. The 2024 AO/AAP consensus provides a framework for managing peri-implant diseases, ranging from nonsurgical debridement to complex surgical procedures [55].
Peri-Implant Disease Management Pathway
Experimental Protocol: Professional Mechanical Debridement for Peri-Implant Mucositis
Objective: To assess the clinical outcomes of nonsurgical debridement on peri-implant mucositis. Methodology:
- Patient Selection: Include patients with peri-implant mucositis (BOP, no bone loss).
- Baseline Measurements: Record BOP, PD, plaque index, and mucosal inflammation.
- Intervention: Perform thorough debridement using titanium, plastic, or ultrasonic scalers designed for implants. Clean the implant surface and prosthesis.
- Oral Hygiene Reinforcement: Re-instruct the patient on personalized home care techniques.
- Follow-up: Re-evaluate clinical parameters at 4, 12, and 24 weeks post-therapy to determine treatment success and stability.
The Researcher's Toolkit: Essential Reagents and Materials
The table below details key materials and their applications in preclinical research on peri-implantitis and its prevention [52].
| Research Reagent / Material | Function in Experimental Models |
|---|---|
| Titanium Alloy Discs | Serve as in-vitro substrates for studying biofilm formation, implant surface decontamination efficacy, and material-biocompatibility interactions. |
| Polymer-based Probes (e.g., Plastic) | Used in clinical and preclinical models to assess peri-implant probing depth and bleeding without causing surface damage to the implant. |
| Chlorhexidine Gluconate Solution | A standard antimicrobial agent used in research to evaluate its efficacy in reducing bacterial load in the peri-implant sulcus and as a surface decontaminant. |
| Biofilm Reactors | Laboratory systems used to grow standardized, reproducible oral biofilms on implant surfaces for testing antimicrobial agents and cleaning protocols. |
| Specialized Scalers (Titanium, Plastic) | Instruments used in experimental protocols for professional debridement to study their cleaning efficacy and potential for altering implant surface topography. |
Frequently Asked Questions (FAQs)
Q1: What is the single most critical risk factor for developing peri-implantitis? A1: A history of periodontitis is one of the most significant risk factors. Other key systemic and behavioral risks include smoking, uncontrolled diabetes, and poor microbial biofilm control [55].
Q2: How often should patients with dental implants attend professional maintenance visits? A2: While intervals should be personalized, supportive peri-implant therapy should be scheduled every 3 to 4 months initially, as biofilm re-colonization occurs within this timeframe. Intervals may be extended to 6 months once long-term stability is established [52] [73].
Q3: Are electric toothbrushes safe and effective for cleaning dental implants? A3: Yes, electric toothbrushes are not only safe but can be particularly effective for plaque removal around implants and are gentle on gum tissue, making them a recommended tool for daily home care [72].
Q4: What is the primary goal of surgical intervention in peri-implantitis? A4: Surgical procedures, which can be resective or reconstructive, aim to decontaminate the implant surface, eliminate the inflammatory lesion, and, in the case of reconstructive surgery, regenerate the lost supporting bone [55].
Q5: Why is a water flosser often recommended for implant maintenance? A5: Water flossers are highly effective at dislodging food particles and plaque from underneath implant-supported bridges and from the challenging contours where the implant meets the gum tissue, areas that are difficult to clean with traditional floss alone [72] [73].
Evaluating Long-Term Efficacy Through Clinical Outcomes and Advanced Metrics
Longitudinal Studies on Reconstructive Therapy Outcomes and Stability Beyond 2 Years
FAQs: Long-Term Outcomes of Reconstructive Peri-Implantitis Therapy
FAQ 1: What is the long-term stability of surgical reconstructive therapy for peri-implantitis beyond 2 years? Evidence from a randomized clinical trial with a 30-month follow-up indicates that surgical reconstructive therapy can demonstrate sustained or even improved stability in most cases. However, about 25% of implants, particularly those with severe peri-implantitis and limited keratinized tissue (<2 mm), may fail within 2 years post-surgery. Clinical parameters such as Peri-implant Pocket Depth (PPD) reduction can be maintained or improved, with one study showing PPD changes from -2.65 mm at 6 months to -3.04 mm at 30 months in a test group receiving adjunctive Er:YAG laser therapy [75].
FAQ 2: Which patient or site-level factors predict poorer long-term outcomes after reconstructive surgery? Key factors associated with higher risk of implant failure or compromised outcomes include:
- Severe peri-implantitis at baseline: Implants with advanced disease are more likely to fail [75].
- Inadequate keratinized tissue: The presence of no or limited keratinized tissue (< 2 mm) is a significant risk factor [75].
- History of periodontitis: This is a major systemic risk indicator for the development and progression of peri-implantitis [55].
- Uncontrolled diabetes and smoking: These systemic and behavioral factors play a pivotal role in the onset and progression of peri-implant diseases [55].
- Surgical complications: Events like membrane exposure during healing are associated with explantation [75].
FAQ 3: How effective are adjunctive technologies, like lasers, for improving long-term results? The use of adjunctive decontamination methods can influence long-term stability. One study reported that the group treated with an Er:YAG laser showed statistically significant improvements in PPD reduction from 6 to 30 months, whereas the control group did not. This suggests that certain adjunctive technologies may favor better outcomes in the longer term [75]. The 2024 AO/AAP consensus notes that implant surface decontamination methods are a key consideration within structured surgical interventions [55].
FAQ 4: What are the critical methodological components of a long-term study on reconstructive therapy? A robust longitudinal study should include:
- Standardized Definitions: Clear case definitions for peri-implant health, mucositis, and peri-implantitis, ideally based on an international consensus [76].
- Clinical and Radiographic Assessment: Repeated, calibrated measurements of PPD, bleeding on probing (BOP), and Radiographic Marginal Bone Level (RMBL) changes at defined intervals (e.g., 6, 12, 24, 30 months) [75].
- Randomized Design: When possible, use a randomized controlled trial (RCT) methodology to compare different surgical protocols or adjunctive therapies [75].
- Strict Inclusion/Exclusion Criteria: Particularly regarding patient history (e.g., periodontitis status) and oral hygiene standards [76].
- Long-Term Follow-up: A follow-up period of at least 2-3 years is necessary to assess stability, with many studies extending to 5 years or more [55].
Experimental Protocols for Long-Term Outcome Studies
Protocol 1: Surgical Reconstructive Therapy with Adjunctive Laser Decontamination
This protocol is derived from a clinical trial that reported outcomes at 30 months [75].
1. Patient Selection and Pre-Surgical Therapy
- Inclusion: Patients diagnosed with peri-implantitis, exhibiting bone loss beyond crestal bone remodeling.
- Exclusion: Uncontrolled systemic diseases, pregnancy, lactation, or allergy to materials used.
- Pre-operative Phase: All patients undergo non-surgical debridement and oral hygiene reinforcement until optimal plaque control is achieved.
2. Surgical Procedure
- Anesthesia and Access: Administer local anesthesia. Raise full-thickness mucoperiosteal flaps to fully expose the defect.
- Granulation Tissue Removal: Debride the implant surface and defect area thoroughly of all granulation tissue.
- Randomization and Decontamination: Randomize sites to receive one of two decontamination protocols:
- Test Group (Laser): Decontaminate the implant surface using an Er:YAG laser with specific settings (e.g., 100 mJ/pulse, 10 Hz, under water irrigation).
- Control Group (Mechanical): Decontaminate using mechanical ablation with titanium or plastic curettes, followed by saline irrigation.
- Reconstructive Material: Apply a bone grafting material (e.g., a xenograft) to the intrabony defect.
- Membrane Placement and Closure: Cover the graft with a collagen membrane. Suture the flaps for primary closure.
3. Post-Surgical Care and Maintenance
- Medication: Prescribe antibiotics and analgesics. Patients use a 0.12% chlorhexidine mouthwash for several weeks.
- Suture Removal: Remove sutures after 10-14 days.
- Supportive Peri-Implant Care (SPC): Patients enter a strict SPC program with professional recalls every 3-6 months.
4. Outcome Assessment
- Primary Outcome: Change in Peri-implant Pocket Depth (PPD).
- Secondary Outcomes: Changes in Radiographic Marginal Bone Level (RMBL), Bleeding on Probing (BOP), and implant survival rate.
- Time Points: Assess at baseline, 6 months, 12 months, 24 months, and 30+ months post-surgery.
Protocol 2: Standardized Clinical and Radiographic Examination for Longitudinal Monitoring
This protocol outlines the methodology for consistent data collection during follow-up visits, crucial for reliable longitudinal data [75] [76].
1. Clinical Examination
- Probing Pocket Depth (PPD): Use a periodontal probe with a controlled force (e.g., 0.25 N) to measure PPD at 6 sites per implant (mesio-buccal, mid-buccal, disto-buccal, mesio-lingual, mid-lingual, disto-lingual). Record to the nearest millimeter.
- Bleeding on Probing (BOP): Note the presence or absence of bleeding within 30 seconds of probing at each site.
- Plaque Score: Record the presence of visible plaque at each implant surface.
- Suppuration: Note the presence or absence of suppuration.
- Mucosal Recession: Measure from the implant shoulder to the mucosal margin.
- Keratinized Tissue Width: Measure in millimeters.
2. Radiographic Examination
- Standardized Technique: Use long-cone parallel technique periapical radiographs. Ensure reproducibility by using custom bite blocks.
- Bone Level Measurement: Measure the distance from the implant reference point (e.g., implant shoulder) to the most coronal level of bone-to-implant contact at mesial and distal aspects. Calibrate measurements using the known distance of implant threads.
3. Patient-Reported Outcomes
- Satisfaction: Record patient satisfaction with the prosthesis and soft tissue aesthetics using a visual analog scale (VAS) or questionnaire [76].
Table 1: Changes in Clinical and Radiographic Parameters Over 30 Months Following Reconstructive Surgery [75]
| Parameter / Group | Baseline | 6 Months | 24 Months | 30 Months | Notes |
|---|---|---|---|---|---|
| PPD Reduction (mm) | |||||
| Control Group | - | -1.85 | - | -1.84 | Change not statistically significant from 6 to 30 months |
| Laser Test Group | - | -2.65 | - | -3.04 | Statistically significant improvement from 6 to 30 months |
| Radiographic Bone Loss (mm) | |||||
| Control Group | - | -1.10 | -1.96 | - | Statistically different from baseline |
| Laser Test Group | - | -1.46 | -2.82 | - | Statistically different from baseline |
| Implant Survival | |||||
| Overall Cohort | 24 implants | - | 18 implants retained | - | 25% failure rate (6 explantations) at ~2 years |
Table 2: Key Risk Factors and Their Impact on Long-Term Outcomes [75] [55]
| Risk Factor / Indicator | Impact on Long-Term Outcome | Clinical Relevance |
|---|---|---|
| Limited Keratinized Tissue (<2mm) | Strongly associated with implant failure; all six explanted implants in one study had this feature [75]. | Consider soft tissue augmentation procedures prior to or during reconstructive surgery. |
| History of Periodontitis | Major risk indicator for the development of peri-implantitis and potentially poorer treatment outcomes [55]. | Requires stringent pre-operative therapy and a lifelong SPC program. |
| Smoking | Contributes to disease onset and progression; reduces healing capacity [55]. | Smoking cessation counseling is a critical component of therapy. |
| Surgical Complication (Membrane Exposure) | Associated with failed cases requiring explantation [75]. | Meticulous surgical technique and post-op care are essential to prevent exposure. |
Research Reagent Solutions and Essential Materials
Table 3: Key Materials and Reagents for Reconstructive Peri-Implantitis Research
| Item | Function in Research / Clinical Protocol |
|---|---|
| Er:YAG Laser | Used as an adjunctive method for implant surface decontamination during surgical therapy. Its efficacy compared to other methods is a key research question [75]. |
| Bone Grafting Material (Xenograft) | The reconstructive material used to fill the intrabony defect following surface decontamination, aiming to regain lost supporting bone [75]. |
| Collagen Membrane | A barrier membrane used in Guided Bone Regeneration (GBR) to cover the bone graft, preventing soft tissue ingrowth and promoting selective bone regeneration [75]. |
| Standardized Periodontal Probe | A calibrated instrument essential for collecting reliable, reproducible clinical measurements (PPD, BOP) at follow-up intervals [76]. |
| Chlorhexidine Mouthwash (0.12%) | A chemical plaque control agent used post-surgically to maintain oral hygiene and reduce the risk of early infection [75]. |
Experimental Workflow and Decision Pathways
Research Workflow for a Long-Term Outcome Study
Clinical Decision Pathway for Managing Peri-Implantitis
Comparative Analysis of Surgical vs Non-Surgical Intervention Success Rates
Peri-implantitis is a destructive inflammatory process affecting the soft and hard tissues surrounding dental implants, characterized by bacterial plaque accumulation that triggers inflammation, bleeding, and progressive bone loss [77]. With a reported prevalence of 22–45% at the patient level, peri-implantitis represents the leading cause of late dental implant failure and poses a significant challenge in implant dentistry [78]. The management of this condition primarily revolves around decontaminating the implant surface, with therapeutic approaches ranging from non-surgical debridement to various surgical interventions [55]. This technical guide provides researchers and clinicians with evidence-based methodologies, comparative success data, and practical protocols for investigating and implementing both surgical and non-surgical treatments within research and clinical settings.
Frequently Asked Questions (FAQs)
Q1: What defines "success" in peri-implantitis interventions, and how are outcomes measured? Success in peri-implantitis treatment is multi-factorial. Key outcomes include:
- Survival Rate: The mere presence of the implant at follow-up [79].
- Clinical Success: Resolution of inflammation (bleeding on probing), reduction in probing depths, and absence of further bone loss [55].
- Radiographic Bone Level: Measured on standardized radiographs to assess bone stability or gain [80].
- Patient-Reported Outcomes: Including satisfaction, absence of pain, and improved quality of life [79].
Q2: What are the primary indications for choosing non-surgical over surgical management? Non-surgical therapy is typically the first-line intervention, indicated for:
- Early-stage peri-implantitis (peri-implant mucositis) [55].
- Superficial bone defects with manageable defect morphology [78].
- As a preliminary step before surgical intervention in more advanced cases [55].
- Cases where patient comorbidities or preferences preclude surgical approaches.
Q3: How does defect morphology influence the choice and success of decontamination methods? Defect morphology critically determines cleaning efficacy. Narrow, deep defects (e.g., 30° configurations) limit instrument access and maneuverability, resulting in poorer cleaning outcomes compared to wider defects (e.g., 60° configurations) [78]. Non-surgical therapy often proves insufficient for complex defects, necessitating surgical access for predictable decontamination [78].
Q4: What are the key risk factors for peri-implantitis that should be controlled in study populations? Consensus reports identify several key risk factors that must be accounted for in research design [55]:
- History of severe periodontitis
- Poor microbial biofilm control
- Smoking
- Uncontrolled diabetes
- Implant malposition and unfavorable prosthetic design
Troubleshooting Common Experimental & Clinical Challenges
Problem: Inconsistent Decontamination Efficacy Across Operator Skill Levels
- Challenge: Significant variability in cleaning outcomes based on operator experience [78].
- Solution: Utilize ultrasonic scalers and titanium brushes, which demonstrate higher consistency across different operator skill levels compared to titanium curettes and air-abrasion devices [78]. Standardized training protocols for all operators are essential.
Problem: Recurrence of Inflammation After Non-Surgical Therapy
- Challenge: Despite initial improvement, disease recurrence is common, especially in advanced cases.
- Solution: Implement strict supportive maintenance programs post-therapy. For advanced defects, consider non-surgical debridement as a pre-treatment to surgical intervention rather than a definitive solution [55].
Problem: Managing Deep and Complex Bone Defects
- Challenge: Non-surgical access is insufficient for effective decontamination of complex defect geometries [78].
- Solution: Transition to surgical protocols (resective or reconstructive) to gain direct access to the contaminated implant surface. The choice between non-reconstructive and reconstructive surgery depends on the aesthetic demands and defect morphology [55].
Comparative Success Rate Data
Table 1: Comparative Outcomes of Surgical vs. Non-Surgical Peri-Implantitis Interventions
| Outcome Measure | Non-Surgical Therapy | Surgical Therapy | Notes & Context |
|---|---|---|---|
| Cleaning Efficacy | 22.9% - 37.2% [78] | Not directly quantified in results | Highly dependent on instrument and defect type |
| Instrument Efficacy (Non-Surgical) | Titanium Brush: 37.2%Ultrasonic Scaler: 35.0%Titanium Curette: 28.1%Air Abrasion: 22.9% [78] | N/A | Varies with operator experience and defect morphology |
| Operator Dependency | High variability (26.1% - 36.6% efficacy range based on experience) [78] | Presumed high | Expert operators achieve superior outcomes |
| Defect Morphology Impact | 60° defects most amenable; 30° and 90° more challenging [78] | Designed for complex defects | Surgical access can overcome non-surgical limitations |
| Therapeutic Goal | Initial inflammation control, non-invasive first step [55] | Definitive defect management | Surgical includes resective and reconstructive approaches |
Table 2: Overall Dental Implant Survival & Failure Context
| Parameter | Rate | Context |
|---|---|---|
| Overall Implant Failure Rate | 3.1% - 6% [81] [82] | Varies by study population and criteria |
| Failure due to Biological Complications | Predominant cause of late failure [83] | Primarily peri-implantitis |
| Pre-Implantitis Risk Factor | History of Periodontitis: ~22% develop peri-implantitis [77] | Critical patient selection consideration |
Detailed Experimental Protocols
Protocol 1: Non-Surgical Implant Surface Decontamination
Objective: To mechanically decontaminate the implant surface in a non-surgical model, simulating clinical debridement of peri-implantitis.
Materials & Reagents:
- Dental implants (e.g., 4mm diameter, 13mm length) [78]
- Fuchsia nail polish or similar for standardized biofilm simulation [78]
- Resin blocks for mounting implants
- Silicone-based prosthesis liner (e.g., Ufi Gel) to simulate soft tissue [78]
- Decontamination Instruments:
- Titanium curette (TiCu)
- Ultrasonic scaler (US) with plastic or titanium tip
- Titanium brush (TiBr)
- Air-polishing device with erythritol powder (AirPo)
Methodology:
- Sample Preparation: Mount implants in resin blocks. Create standardized bone defect models (30°, 60°, 90° configurations) around implants.
- Biofilm Simulation: Apply a standardized coating of fuchsia nail polish to the implant surface to simulate biofilm. Cover the defect with silicone to simulate soft tissue.
- Decontamination Procedure:
- Assign operators with varying experience levels (student, experienced hygienist, dentist).
- Provide three minutes per decontamination procedure per instrument type.
- Instruct operators to clean the surface to the best of their ability without knowledge of specific defect configuration.
- Efficacy Assessment:
- Photograph implants post-treatment with a standardized macro photography setup.
- Analyze images using color quantification software (e.g., Image Color Summarizer v0.80) to calculate the percentage of cleaned surface area.
- Statistical Analysis:
- Use two-way ANOVA to compare cleaning efficacy across operator experience, instrument type, and defect morphology.
- Perform post-hoc tests with Bonferroni correction for significant findings.
Protocol 2: Surgical Access & Defect Management
Objective: To evaluate surgical interventions for peri-implantitis through flap elevation, defect debridement, and surface decontamination.
Materials & Reagents:
- Standard surgical kit for periodontal flap surgery
- Bone grafting materials (if reconstructive approach is tested)
- Barrier membranes for guided bone regeneration (GBR)
- Same decontamination instruments as Protocol 1
Methodology:
- Surgical Access:
- Create full-thickness mucoperiosteal flaps to fully expose the bone defect and implant surface.
- Thoroughly degranulate the defect to remove all granulomatous tissue.
- Defect Classification:
- Classify the defect morphology (supracrestal, intrabony, crater-shaped).
- Measure defect dimensions (depth, width, number of implant surfaces affected).
- Implant Surface Decontamination:
- Apply one of the tested decontamination methods (e.g., titanium brush, ultrasonic scaler) to the exposed implant surface.
- Utilize mechanical instrumentation with copious irrigation.
- Surgical Intervention:
- Non-Reconstructive Surgery: Resect and re-contour bone where aesthetics are not a priority.
- Reconstructive Surgery: Apply bone grafting materials and/or barrier membranes to regenerate lost bone.
- Closure: Suture flaps with appropriate suturing technique.
- Post-operative Care: Administer antibiotics and analgesics as needed. Institute supportive maintenance protocol after healing.
Research Reagent Solutions & Essential Materials
Table 3: Essential Research Materials for Peri-Implantitis Intervention Studies
| Item | Function/Application | Example Products/Types |
|---|---|---|
| Titanium Brushes | Mechanical implant surface decontamination; shown to have high efficacy and low operator-dependency [78] | Specific implant-system compatible brushes |
| Ultrasonic Scalers | Mechanical debridement with irrigation; effective and consistent across operators [78] | Plastic or titanium tips to minimize surface alteration |
| Air-Polishing Devices | Chemical-mechanical decontamination using abrasive powders [78] | Erythritol, glycine-based powders |
| Titanium Curettes | Mechanical scaling of implant surfaces; less effective than ultrasonic and brush options [78] | Various shapes/sizes for different implant areas |
| Bone Grafting Materials | Reconstructive surgical procedures to regenerate lost bone [55] | Xenografts, allografts, alloplasts |
| Barrier Membranes | Guided bone regeneration (GBR) in reconstructive surgery [55] | Resorbable & non-resorbable membranes |
| Biofilm Simulation Materials | Standardized in-vitro simulation of clinical biofilm for testing decontamination methods [78] | Fuchsia nail polish, microbial cultures |
Visualization of Treatment Pathways and Experimental Workflows
Figure 1: Clinical Decision Pathway for Peri-Implantitis Management. This flowchart outlines the evidence-based clinical decision process for managing peri-implantitis, from initial diagnosis through non-surgical and surgical interventions to long-term maintenance.
Figure 2: Experimental Workflow for Decontamination Efficacy Testing. This diagram illustrates the standardized in-vitro methodology for evaluating the efficacy of different implant surface decontamination instruments and techniques.
Validation of Microbiome-Based Diagnostic Models for Early Disease Detection
Troubleshooting Guide & FAQs
This technical support resource addresses common challenges in developing and validating microbiome-based diagnostic models for the early detection of peri-implantitis, providing practical solutions for researchers and clinicians.
Frequently Asked Questions
Q: Our microbiome-based model performs well on internal validation but fails on external datasets. What could be the cause? A: This common issue, known as poor cross-study portability, often stems from technical variability between studies rather than biological differences. Key factors include:
- Sequencing protocol differences: Variations in 16S rRNA regions sequenced, library preparation kits, and sequencing platforms create technical batch effects that impair model generalization [84].
- Bioinformatic processing disparities: Different pipelines for taxonomy assignment, contamination removal, and data normalization introduce inconsistencies [85].
- Population heterogeneity: Demographic, geographic, and clinical characteristic variations across cohorts affect microbial community composition [84].
Solution: Implement a harmonized analysis framework using raw sequencing data when possible. Apply ComBat or other batch-effect correction methods, and train models on multiple datasets from diverse populations to improve robustness [84].
Q: What is the optimal sequencing method for microbiome-based diagnostics in peri-implantitis? A: The choice depends on your specific diagnostic goals and resources:
Table: Sequencing Method Comparison for Peri-Implantitis Diagnostics
| Method | Resolution | Advantages | Limitations | Best For |
|---|---|---|---|---|
| 16S rRNA Sequencing | Genus to species level | Cost-effective; standardized protocols; suitable for large cohorts | Limited functional insights; cannot resolve strains | Initial microbial community screening; large-scale studies [86] |
| Shotgun Metagenomics | Species to strain level | Identifies all microbial domains; reveals functional potential; detects AR genes | Higher cost; complex bioinformatics | Comprehensive pathogen detection; resistance profiling [87] |
| Metatranscriptomics | Functional activity | Reveals actively expressed pathways; identifies true metabolic activity | RNA instability; technically demanding; high cost | Understanding active disease mechanisms; biomarker validation [5] |
Q: Which machine learning algorithm is most effective for microbiome-based classification of peri-implantitis? A: Algorithm performance varies by dataset, but random forest and ridge regression have demonstrated consistent performance:
- Random Forest: Effectively handles high-dimensional, compositional data without requiring normalization; provides feature importance rankings helpful for biomarker discovery [87] [85].
- Ridge Regression: Performs well with correlated features common in microbiome data; reduces overfitting through L2 regularization [84].
- Support Vector Machines: Useful with non-linear relationships but may require extensive parameter tuning [85].
Critical consideration: Algorithm choice matters less than proper validation strategy. Always use leave-one-study-out cross-validation or independent external validation to assess true clinical utility [84].
Q: How can we identify meaningful biomarkers from thousands of microbial features? A: Employ a multi-stage feature selection approach:
- Pre-filtering: Remove low-prevalence taxa (e.g., present in <5% of samples) [84].
- Univariate analysis: Identify features with significant abundance changes (account for multiple testing).
- Multivariate selection: Use embedded methods like LASSO or recursive feature elimination that consider feature interactions [88].
- Biological validation: Correlate candidate biomarkers with clinical parameters and validate in independent cohorts [87] [5].
Q: Our models struggle to distinguish peri-implantitis from periodontitis. How can we improve specificity? A: This is a recognized challenge due to shared pathogenic bacteria. Strategies to improve discrimination include:
- Incorporate strain-level resolution: Shotgun metagenomics can reveal distinct Fusobacterium nucleatum subspecies associated with peri-implantitis versus periodontitis [87].
- Add functional profiling: Metatranscriptomics can identify disease-specific active pathways despite taxonomic similarities [5].
- Include host response markers: Integrate host transcriptional biomarkers (e.g., IL1B, CXCL8) with microbial features for enhanced specificity [88].
Experimental Protocols for Method Validation
Protocol 1: Cross-Study Validation Framework
Purpose: To evaluate model performance across diverse populations and technical conditions.
Procedure:
- Data Collection: Aggregate at least 3-5 independent datasets with raw sequencing data available [84].
- Harmonized Processing:
- Model Training:
- Train separate models on each individual dataset.
- Train a combined model on merged datasets after batch-effect correction.
- Validation:
- Perform internal validation via 10-fold cross-validation on each dataset.
- Conduct external validation by testing each study-specific model on all other datasets.
- Evaluate combined model via leave-one-study-out cross-validation [84].
Success Metrics: Cross-study AUC >0.70 indicates reasonable generalizability [84].
Protocol 2: Biomarker Validation Workflow
Purpose: To verify putative diagnostic biomarkers in independent cohorts.
Procedure:
- Discovery Phase:
- Technical Validation:
- Verify biomarkers using different methodological approach (e.g., if discovered via 16S, validate with qPCR or shotgun sequencing).
- Assess stability across sample collection methods (e.g., curette vs. paper point).
- Biological Validation:
- Test association with clinical severity metrics (PD, BOP, bone loss).
- Assess specificity against relevant controls (health, mucositis, periodontitis) [88].
- Functional Validation:
- For functional biomarkers, validate using metatranscriptomics or metabolomics [5].
Diagnostic Performance Data
Table: Performance Metrics of Microbiome-Based Diagnostic Models for Peri-Implantitis
| Model Type | Sequencing Method | Sample Size | Internal AUC | External AUC | Key Biomarkers |
|---|---|---|---|---|---|
| Random Forest Classifier | Shotgun Metagenomics | 158 sites, 102 patients | 0.96 (Health vs. Peri-implantitis) | 0.78 (Cross-condition) | 447 bacterial species, including 150 uncharacterized [87] |
| Ridge Regression | 16S rRNA Sequencing | 4489 samples across 22 studies | 0.72 (Average within-study) | 0.61 (Average cross-study) | P. gingivalis, F. nucleatum subsp. vincentii, T. forsythia [84] |
| Integrated Taxonomic + Functional | Full-length 16S + Metatranscriptomics | 48 samples, 32 patients | 0.85 (Health vs. Disease) | N/R | Streptococcus and Rothia species (health); specific enzymes (disease) [5] |
| Meta-Analysis Pooled Performance | Multiple methods | 11 studies | 0.91 (Pooled AUC) | N/R | Immune markers, microbial signatures [88] |
The Scientist's Toolkit: Essential Research Reagents
Table: Key Research Reagents for Microbiome-Based Diagnostic Development
| Reagent/Resource | Function | Examples/Specifications |
|---|---|---|
| MetaPhlAn Database | Taxonomic profiling from shotgun metagenomics | Provides species-level and strain-level classification; Version 4 improves characterization of unknown species [87] |
| HOMD (Human Oral Microbiome Database) | 16S rRNA sequence reference database | Oral-specific taxonomy assignment; essential for oral microbiome studies [86] |
| SIAMCAT R Package | Machine learning for microbiome data | Standardized workflow for feature selection, model training, and validation; supports multiple algorithms [84] |
| PICRUSt2 | Functional prediction from 16S data | Inferrs metabolic pathways from taxonomic data; useful when shotgun sequencing unavailable [86] |
| CIBERSORT | Immune cell deconvolution | Quantifies immune cell infiltration from host transcriptomic data; identifies immune patterns in peri-implantitis [88] |
Experimental Workflow Visualization
Microbiome Diagnostic Development Workflow
Biomarker Integration Pathway
Multi-Omics Biomarker Integration for Diagnosis
▢ Frequently Asked Questions (FAQs)
FAQ 1: What is the long-term economic evidence for comparing tooth preservation to implant placement? A 2025 retrospective study with a mean follow-up of 6.4 years provides direct comparative data. The study found that while survival rates were comparable, the total complication rate was significantly higher in the implant group (26.1%) compared to the periodontal regeneration group (9.1%), largely due to peri-implantitis. The cost-effectiveness of implants was highly dependent on the initial tooth prognosis. For teeth with a good prognosis (Periodontal Risk Score of 1), periodontal regeneration was more cost-effective. However, for teeth with a poor prognosis, such as those with a one-wall defect and furcation involvement (which increased tooth loss risk fourfold), implant placement became a more cost-effective option [89].
FAQ 2: Which specific oral self-care practices are most cost-effective for preventing peri-implant diseases? A 2025 systematic review identified several cost-effective, evidence-based self-care strategies. These represent a low-cost investment to prevent expensive professional treatments for peri-implantitis [90].
- Toothbrushes: Powered toothbrushes, particularly oscillating-rotating models, are more effective at reducing plaque and inflammation than manual brushes [90].
- Toothpastes: Toothpastes containing triclosan consistently outperform fluoride-only toothpastes in reducing plaque, bleeding on probing, and pathogenic bacteria [90].
- Interdental Aids: Interproximal brushes and oral irrigators are more effective than floss at reducing inflammatory markers between implants [90].
- Mouth Rinses: Stannous fluoride-based rinses show anti-inflammatory benefits, while prolonged use of chlorhexidine may elevate inflammatory cytokines and is not typically recommended for long-term maintenance [90].
FAQ 3: From a health economics perspective, what is the most critical period for implementing preventive strategies? Prevention must begin before an implant is ever placed. A history of periodontitis is a strong risk factor for future peri-implantitis. Therefore, the most cost-effective strategy is to preserve natural teeth whenever possible, as the initial cost of periodontal treatment is often lower than the long-term costs of managing peri-implant complications. Prevention then continues indefinitely through regular, risk-based supportive care [91].
▢ Troubleshooting Guides
Issue 1: High Complication Rates in a Patient Cohort Problem: A clinical study arm shows a higher-than-expected rate of peri-implantitis, threatening the cost-effectiveness of the implant protocol. Investigation & Resolution:
- Step 1: Check Patient Risk Stratification. Verify that patients were stratified based on their periodontal risk score (PRS) and history of periodontitis. Data shows that PRS of 2 and 3 are associated with a 22 to 35-fold greater risk of complications compared to PRS1 [89].
- Step 2: Audit Oral Hygiene Protocols. Ensure the study utilizes a multimodal self-care regimen. The most effective protocol combines mechanical and chemical methods (e.g., powered toothbrushing, interdental aids, and therapeutic toothpaste) [90].
- Step 3: Evaluate Recall Intervals. Supportive Peri-Implant Therapy (SPiT) with regular, risk-based recall visits is essential. Implement a monitoring schedule where high-risk patients are seen more frequently [92].
Issue 2: Inconsistent Results in Testing Novel Anti-Biofilm Implant Coatings Problem: An in-vitro experiment on a new bioactive coating shows inconsistent biofilm inhibition results. Investigation & Resolution:
- Step 1: Standardize the Biofilm Formation Model. Use a validated, continuous-flow model that simulates the oral environment rather than static assays. Ensure the use of a polymicrobial consortium that includes known peri-implantopathogens.
- Step 2: Control the Surface Characterization. Use atomic force microscopy (AFM) and scanning electron microscopy (SEM) to verify the consistency and topography of the coating on each implant sample. Surface roughness is a critical variable.
- Step 3: Validate the Decontamination Assay. When testing the coating's resistance to cleaning, use a standardized debridement protocol. A promising reference method is electrolytic decontamination (e.g., GalvoSurge), which gently generates hydrogen peroxide to neutralize biofilm and may serve as an effective benchmark [91].
The table below synthesizes key quantitative data from recent studies to aid in the economic modeling of preventive strategies.
Table 1: Comparative Long-Term Outcomes of Tooth Preservation vs. Implant Placement
| Metric | Periodontal Regeneration (PR) | Dental Implant (DI) | Notes |
|---|---|---|---|
| Survival Time (Mean) | 9.3 years [89] | 12.65 years [89] | Based on Kaplan-Meier analysis. |
| Total Complication Rate | 9.1% [89] | 26.1% [89] | Primary complication for DI is peri-implantitis. |
| Risk Factor: Furcation Involvement | 4x increased risk of tooth loss [89] | Not Applicable | Informs patient selection for PR. |
| Risk Factor: Periodontal Risk Score (PRS) | PRS 2 & 3: 22-35x greater risk than PRS 1 [89] | Not Applicable | Critical for risk stratification. |
Table 2: Efficacy of Self-Care Modalities for Preventing Peri-Implant Diseases
| Preventive Modality | Key Finding / Efficacy | Evidence Level |
|---|---|---|
| Powered Toothbrushes | Superior plaque and inflammation reduction vs. manual brushes [90]. | Systematic Review |
| Triclosan Toothpaste | Outperforms fluoride-only formulations in reducing plaque, bleeding, and bacteria [90]. | Systematic Review |
| Interdental Brushes/Oral Irrigators | More effective than floss for reducing interproximal inflammation [90]. | Systematic Review |
| Stannous Fluoride Rinse | Shows potential anti-inflammatory benefits [90]. | Systematic Review |
▢ Experimental Protocols
Protocol 1: Clinical Trial Protocol for Evaluating a Supportive Peri-Implant Therapy (SPiT) Program This protocol outlines a method to test the cost-effectiveness of a structured maintenance program.
- Patient Recruitment and Stratification: Recount patients with dental implants but without active peri-implantitis. Stratify them into risk groups based on history of periodontitis, smoking status, and systemic health [89] [91].
- Intervention Arm (Structured SPiT): Assign the intervention group to a structured SPiT program. This includes:
- Professional Mechanical Debridement: Using titanium or plastic scalers to avoid implant surface damage [92].
- Reinforcement of Personalized Oral Hygiene: Prescribing a multimodal regimen based on individual needs (e.g., powered toothbrush, interdental brushes, stannous fluoride rinse) [90].
- Risk-Based Recall Schedule: High-risk patients recall every 3-4 months; low-risk patients every 6 months [92].
- Control Arm (Standard Care): The control group receives routine care with annual recall and non-standardized hygiene advice.
- Outcome Measures:
- Primary: Incidence of peri-implant mucositis progressing to peri-implantitis over a 5-year period.
- Secondary: Cost per quality-adjusted tooth year (QATY), patient-reported comfort, and plaque/bleeding indices.
- Economic Analysis: Calculate the Incremental Cost-Effectiveness Ratio (ICER) to determine the cost per case of peri-implantitis prevented in the SPiT arm [89].
Protocol 2: In-Vitro Model for Testing Novel Bioactive Coatings This protocol describes a standardized assay for screening the efficacy of implant surface modifications.
- Surface Coating and Characterization: Apply the experimental coating to grade 4 titanium or Ti-Zr alloy discs. Characterize the surface using SEM and AFM to confirm uniformity, roughness, and coating thickness [93].
- Biofilm Formation: Inoculate the coated discs in a continuous-flow bioreactor with a defined polymicrobial consortium including Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans. Allow biofilm formation for 72-96 hours.
- Biofilm Viability Assessment (Intervention): Use the bioreactor's effluent to apply a simulated, non-invasive cleaning cycle with an electrolytic decontamination system (e.g., GalvoSurge) as a positive control against mechanical debridement alone [91].
- Analysis:
- Microscopy: Use confocal laser scanning microscopy (CLSM) with live/dead staining (e.g., SYTO 9/propidium iodide) to quantify the percentage of dead bacteria in the biofilm.
- Molecular Analysis: Perform qPCR to quantify the biomass of specific peri-implantopathogens remaining on the disc surface.
- Cytocompatibility: Seed human osteoblast-like cells (e.g., MG-63) on the coated discs to assess post-decontamination cell viability and proliferation.
▢ Research Workflow and Pathways
The following diagram illustrates the logical workflow for planning and analyzing research on the cost-effectiveness of preventive strategies for peri-implantitis.
▢ The Scientist's Toolkit: Key Research Reagent Solutions
Table 3: Essential Materials for Peri-Implantitis Prevention Research
| Item | Function in Research | Example / Note |
|---|---|---|
| Ti-Zr Alloy Implant Discs | Substrate for testing novel coatings and surface treatments. Provides enhanced strength for narrow-diameter implants [93]. | Straumann Roxolid [93] |
| Polymicrobial Biofilm Consortium | Creates a clinically relevant in-vitro model for testing anti-biofilm efficacy of materials or drugs. | Includes P. gingivalis, A. actinomycetemcomitans. |
| Electrolytic Decontamination Unit | Serves as a benchmark for non-invasive biofilm removal in experiments. Generates hydrogen peroxide to lift and neutralize biofilm without damaging titanium [91]. | Straumann GalvoSurge [91] |
| Oscillating-Rotating Powered Toothbrush | Essential for in-vitro or clinical studies validating mechanical plaque removal protocols. Proven superior to manual brushing in evidence-based reviews [90]. | Oral-B (as cited in research) [90] [91] |
| Confocal Laser Scanning Microscope (CLSM) | Enables 3D visualization and quantification of biofilm viability on implant surfaces after intervention, using live/dead stains [93]. | |
| qPCR Assays for Peri-implantopathogens | Quantifies specific bacterial load from biofilm models or clinical samples (e.g., peri-implant crevicular fluid) to measure preventive intervention efficacy [90]. | Targets for P. gingivalis and A. actinomycetemcomitans. |
This technical support center provides troubleshooting guides and FAQs to assist researchers in validating biomarkers for predicting peri-implantitis progression.
Frequently Asked Questions (FAQs)
FAQ 1: What defines a clinically useful predictive biomarker for peri-implantitis? A predictive biomarker should not only correlate with the clinical endpoint but also forecast future disease progression in the intended patient population. Its performance must be reproducible in independent test cohorts. The intended use, such as identifying at-risk patients before irreversible bone loss occurs, guides the required level of validation evidence [94].
FAQ 2: My multi-omics model performs well on my cohort but fails in an independent validation set. What are potential causes? This often stems from overfitting or cohort-specific biases. High-dimensional omics data (e.g., metatranscriptomics) with small sample sizes (the "p >> n" problem) is particularly susceptible [95]. Mitigation strategies include:
- Adequate Power: Use sample size determination methods in the study design phase [95].
- Robust Validation: Ensure performance is reproducible in independent test cohorts from different populations or geographic regions [96] [94].
- Data Integration: Assess if omics data provides value beyond traditional clinical markers (e.g., plaque index) by conducting comparative evaluations [95].
FAQ 3: How can I improve the reliability of my biomarker discovery from high-throughput data?
- Ensure Data Quality: Implement data type-specific quality controls (e.g., using tools like fastQC for NGS data or Normalyzer for proteomics data) and standardize formats using established guidelines (e.g., MIAME for microarray data) [95].
- Effective Preprocessing: Apply adequate filtering to remove uninformative features and address systematic biases through transformation and normalization [95].
- Machine Learning: Use feature selection algorithms (e.g., LASSO, SVM-RFE, Boruta) to identify the most robust biomarker candidates and reduce overfitting [88] [97].
FAQ 4: What is the process for translating a discovered biomarker into a clinically validated diagnostic? The transition from discovery to a validated in vitro diagnostic (IVD) product involves several stages [94]:
- Research Use Only (RUO): Initial test method development and small-scale validation to gain confidence.
- Retrospective Clinical Validation: Analysis of stored patient samples to gather additional evidence on performance.
- Investigational Use: Use of the biomarker to inform patient treatment decisions within clinical trials.
- Validation for Marketing Approval: Conducting rigorous analytical and clinical validation studies to meet regulatory standards (e.g., from the FDA or under EU IVDR).
Troubleshooting Experimental Protocols
Protocol 1: Metatranscriptomic Analysis of Peri-Implant Biofilms
This protocol is for identifying taxonomic and functional biomarkers from biofilm samples [96].
| Workflow Stage | Key Reagents & Kits | Potential Issue & Solution |
|---|---|---|
| Sample Collection | Sterile curettes, paper points, RNA stabilization buffer | Issue: Low RNA yield/biomass. Solution: Use a tailored co-isolation protocol; immediately stabilize RNA; discard samples contaminated with saliva or blood [96]. |
| RNA Sequencing | Illumina platform, library prep kits | Issue: High technical noise. Solution: Perform quality checks (e.g., fastQC); use variance-stabilizing transformations during preprocessing [96] [95]. |
| Bioinformatic Analysis | Tailored genomic reference database, enzyme annotation databases | Issue: Low taxonomic resolution. Solution: Utilize a custom database encompassing thousands of genomes or metagenome-assembled genomes for higher resolution [96]. |
| Functional Validation | Machine learning algorithms (e.g., LASSO, SVM-RFE) [97] | Issue: Model overfitting. Solution: Apply feature selection algorithms (e.g., LASSO, SVM-RFE) and validate predictive accuracy (AUC) in a separate cohort [96] [88]. |
Protocol 2: Multiplex Immunoassay of Peri-Implant Crevicular Fluid (PICF)
This protocol measures concentrations of multiple inflammatory and bone-remodeling biomarkers (e.g., APRIL, RANKL, IL-23) from PICF [98].
| Workflow Stage | Key Reagents & Kits | Potential Issue & Solution |
|---|---|---|
| PICF Collection | Paper strips (e.g., Periopaper), 1.5mL Eppendorf tubes, -80°C freezer | Issue: Variable sample volume. Solution: Standardize sampling time (e.g., 30 seconds per site); pool strips from multiple sites per implant; carefully remove supramucosal plaque first [98]. |
| Sample Elution | PBS buffer, protease inhibitor cocktail | Issue: Protein degradation. Solution: Add protease inhibitor to elution buffer; keep samples on ice during elution; centrifuge to remove debris [98]. |
| Multiplex Assay | Luminex assay kits, magnetic beads, detection antibodies | Issue: Poor assay precision. Solution: Validate the assay per manufacturer's guidelines; include appropriate positive and negative controls; ensure statistical power in study design [94] [98]. |
| Diagnostic Accuracy | Statistical software for ROC analysis | Issue: Low diagnostic specificity. Solution: Combine biomarker levels with clinical parameters (e.g., plaque index) in a regression model to enhance accuracy (AUC) [98]. |
The Scientist's Toolkit: Research Reagent Solutions
| Item | Function / Application |
|---|---|
| Paper Strips | Standardized collection of Peri-Implant Crevicular Fluid (PICF) for downstream immunoassays [98]. |
| RNA Stabilization Buffer | Preserves RNA integrity in biofilm samples from collection to nucleic acid extraction for metatranscriptomics [96]. |
| Luminex Multiplex Kits | Allows simultaneous quantification of multiple cytokines (e.g., IL-1β, TNF-α, APRIL, RANKL) from a single, small-volume PICF sample [98]. |
| Next-Generation Sequencing (NGS) | Provides comprehensive profiling of microbial communities (16S rRNA) and functional activities (RNAseq) in biofilms [96] [99]. |
| Custom Genomic Database | A curated database of thousands of bacterial genomes essential for high-resolution taxonomic classification in metatranscriptomic studies [96]. |
Table 1: Diagnostic Performance of Select Peri-Implantitis Biomarkers
| Biomarker Category | Specific Biomarker | Sample Source | Predictive Value (AUC) | Key Findings |
|---|---|---|---|---|
| Host Inflammatory | APRIL [98] | PICF | 0.95 (when combined with Plaque Index) | Significantly elevated in peri-implantitis; high sensitivity (94.4%) and negative predictive value (96.8%) [98]. |
| Bone Remodeling | RANKL [98] | PICF | - | Significantly increased in peri-implantitis; positively correlated with clinical attachment loss and bleeding on probing [98]. |
| Microbial Functional | Urocanate hydratase, Tripeptide aminopeptidase [96] | Biofilm (RNAseq) | 0.85 (Integrated model) | Metatranscriptomic biomarkers indicative of amino acid metabolism shifts in disease; enhanced predictive accuracy when combined with taxonomy [96]. |
| Machine Learning Models | Integrated Taxonomic & Functional Signatures [88] | Mixed (Biofilm, Tissue) | 0.91 (Pooled from meta-analysis) | ML models integrating multiple data types show high reliability in distinguishing peri-implantitis from health [88]. |
| Technology / Source | Key Advantages | Common Technical Challenges |
|---|---|---|
| Peri-Implant Crevicular Fluid (PICF) | Site-specific; non-invasive collection; rich in host-derived inflammatory mediators [100] [98]. | Low sample volume; potential contamination; requires sensitive multiplex assays [98]. |
| Biofilm (Metatranscriptomics) | Reveals active microbial community and functional pathways; high-resolution taxonomic data with full-length 16S [96]. | Low biomass; RNA instability; complex bioinformatic workflows; high cost [96]. |
| Multiplex Immunoassays (Luminex) | High-throughput; measures multiple analytes simultaneously from small sample volumes [98]. | Requires specialized equipment; antibody cross-reactivity; dynamic range limitations [98]. |
| Digital Pathology / Image Analysis | Quantitative, objective, and reproducible assessment of tissue biomarkers [101]. | Impact of preanalytical variables (e.g., fixation); costly whole-slide scanners; complex data management [101]. |
Experimental Workflows and Pathways
Biomarker Development Workflow
Peri-Implantitis Bone Loss Pathway
Conclusion
The prevention of peri-implantitis requires an integrated approach combining fundamental understanding of biofilm pathogenesis with advanced diagnostic and therapeutic technologies. Key takeaways include the critical importance of identifying high-risk patients through biomarker profiling and periodontal history, implementing structured supportive care programs with 3-6 month recall intervals, and developing novel biomaterials with sustained antimicrobial properties. Future research should focus on validating non-invasive diagnostic tools using PICF and salivary biomarkers, optimizing local drug delivery systems for long-term protection, and conducting large-scale randomized trials comparing the cost-effectiveness of various preventive protocols. For biomedical researchers, promising directions include engineering next-generation implant surfaces that resist microbial adhesion while promoting osseointegration, developing immunomodulatory approaches to control dysregulated host responses, and creating personalized prevention strategies based on individual microbial and immune profiles.
References
- 1. The Oral Microbiome of Peri-Implant Health and Disease [https://pmc.ncbi.nlm.nih.gov/articles/PMC11506630/]
- 2. AO/AAP consensus on prevention and management of peri ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12273748/]
- 3. Challenges in the Microbiological Diagnosis of Implant ... [https://www.frontiersin.org/journals/microbiology/articles/10.3389/fmicb.2021.750460/full]
- 4. Linking peri-implantitis to microbiome changes in affected ... [https://www.frontiersin.org/journals/cellular-and-infection-microbiology/articles/10.3389/fcimb.2025.1543100/full]
- 5. Integrative microbiome- and metatranscriptome-based ... [https://www.nature.com/articles/s41522-025-00807-6]
- 6. Microbiome Shifts in Peri-Implantitis: Longitudinal ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12361765/]
- 7. Microbiological and functional traits of peri-implant ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC11287996/]
- 8. FAQs: <61> Microbial Examination of Nonsterile Products [https://www.usp.org/frequently-asked-questions/microbial-examination-nonsterile-products-microbial-enumeration-tests]
- 9. Role of immune dysregulation in peri-implantitis - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC11563827/]
- 10. Peri-implant diseases triggered by oral microdysbiosis [https://pmc.ncbi.nlm.nih.gov/articles/PMC12367669/]
- 11. How Can Imbalance in Oral Microbiota and Immune ... [https://www.mdpi.com/1422-0067/24/24/17620]
- 12. Signaling pathways of dental implants' osseointegration [https://www.nature.com/articles/s41405-024-00211-w]
- 13. A Comprehensive Review of Peri-implantitis Risk Factors [https://link.springer.com/article/10.1007/s40496-020-00274-2]
- 14. Intergenerational continuity in periodontal health [https://pmc.ncbi.nlm.nih.gov/articles/PMC3071145/]
- 15. Is Periodontal Disease Hereditary: Unraveling the Genetic ... [https://murphydentalfc.com/periodontal-disease-hereditary/]
- 16. Preventing Implant Failure: Best Practices and Expert Advice [https://www.centralfamilysmiles.com/blog-posts/preventing-implant-failure-best-practices-and-expert-advice]
- 17. Management of peri-implantitis - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC3612185/]
- 18. The Etiology and Management of Dental Implant Failure [https://pmc.ncbi.nlm.nih.gov/articles/PMC9674049/]
- 19. Impact of tribocorrosion and titanium particles release on ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC8488597/]
- 20. Corrosion of titanium under simulated inflammation ... [https://www.sciencedirect.com/science/article/pii/S1742706121006590]
- 21. The systemic and local interactions related to titanium implant ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC11584828/]
- 22. Titanium particles in peri-implantitis [https://pmc.ncbi.nlm.nih.gov/articles/PMC10667540/]
- 23. The unfavorable role of titanium particles released from ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC7961127/]
- 24. All-in-one design of titanium-based dental implant systems ... [https://www.sciencedirect.com/science/article/abs/pii/S014296122500170X]
- 25. Current Data on Oral Peri-Implant and Periodontal ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC9781768/]
- 26. Do Concurrent Peri-Implantitis and Periodontitis Share ... [https://www.mdpi.com/2304-6767/12/4/113]
- 27. Unraveling the Microbial Mosaic and Inflammatory Core of ... [https://pubmed.ncbi.nlm.nih.gov/40676912/]
- 28. Investigation of the relationships between peri-implant ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC11622867/]
- 29. An in vitro study exploring a new method for managing peri ... [https://www.nature.com/articles/s41598-025-08946-8]
- 30. A systematic review of the patient reported outcome ... [https://www.nature.com/articles/s41405-024-00273-w]
- 31. Role of Salivary Biomarkers IL-1β and MMP-8 in Early ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12028559/]
- 32. Role of Salivary Biomarkers IL-1β and MMP-8 in Early ... [https://pubmed.ncbi.nlm.nih.gov/40283051/]
- 33. Prevention and management of peri-implant disease [https://pubmed.ncbi.nlm.nih.gov/37042296/]
- 34. A review of alternative strategies to prevent peri-implantitis ... [https://www.sciencedirect.com/science/article/pii/S1075996421000871]
- 35. Frontiers | Metagenomic and Metatranscriptomic Insight Into Oral Biofilms in Periodontitis and Related Systemic Diseases [https://www.frontiersin.org/journals/microbiology/articles/10.3389/fmicb.2021.728585/full]
- 36. Uncovering complex microbiome activities via ... [https://microbiomejournal.biomedcentral.com/articles/10.1186/s40168-018-0591-4]
- 37. Metatranscriptomic analysis of an in vitro biofilm model ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC6461087/]
- 38. How to study biofilms: technological advancements in ... [https://www.frontiersin.org/journals/cellular-and-infection-microbiology/articles/10.3389/fcimb.2023.1335389/full]
- 39. Metatranscriptomic Profiling of the Subgingival Microbiome ... [https://www.phlox.or.id/index.php/crown/article/view/237]
- 40. Metatranscriptomics-based metabolic modeling of patient-specific urinary microbiome during infection | npj Biofilms and Microbiomes [https://www.nature.com/articles/s41522-025-00823-6]
- 41. Effectiveness of a Local Drug Delivery System Based on ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC11976603/]
- 42. Biological and immunotoxicity evaluation of antimicrobial ... [https://www.nature.com/articles/srep16336]
- 43. Multilayered coating on titanium for controlled release of ... [https://www.sciencedirect.com/science/article/pii/S0142961213004808]
- 44. Long-lasting renewable antibacterial porous polymeric ... [https://www.nature.com/articles/s41467-021-23069-0]
- 45. Antibacterial Peptide HHC-36 Sustained-Release Coating ... [https://www.frontiersin.org/journals/bioengineering-and-biotechnology/articles/10.3389/fbioe.2021.735889/full]
- 46. Local drug delivery agents as adjuncts to endodontic and ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC4034307/]
- 47. Local Antibacterial and Antimicrobial Drug Delivery Systems [https://www.mdpi.com/journal/pharmaceutics/special_issues/DDS_Antibacterial]
- 48. Review Advances on electrochemical disinfection research [https://www.sciencedirect.com/science/article/abs/pii/S0048969723076738]
- 49. Electrolytic Disinfection Systems - Water & Wastewater [https://www.waterandwastewater.com/electrolytic-disinfection-systems/]
- 50. Electrolytic Cleaning of Dental Implants: A Scoping Review of ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12025659/]
- 51. Acid based electrolytic cleaning: FAQs + Q&A Forum [https://www.finishing.com/350/82.shtml]
- 52. Implant Maintenance: A Clinical Update - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC4897104/]
- 53. Recall | Prevention and Treatment of Periodontal Diseases in ... [https://www.periodontalcare.sdcep.org.uk/guidance/dental-implants/general-care-of-dental-implants/recall/]
- 54. Clinically relevant aspects of professional follow-up care for ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12101065/]
- 55. AO/AAP consensus on prevention and management of peri ... [https://pubmed.ncbi.nlm.nih.gov/40501397/]
- 56. Peri-Implantitis Prevention and Treatment [https://gdt-implants.com/blogs/gdt-dental-implants-blog/peri-implantitis-scientific-insights-into-prevention-and-treatment-protocols]
- 57. The Prevention of Implant Surface Alterations in ... [https://www.mdpi.com/1660-4601/17/8/2624]
- 58. Surface Decontamination on the Reconstructive Therapy of ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12281608/]
- 59. Treatment of peri-implant mucositis using an Er:YAG laser ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC11759739/]
- 60. Erythritol airpolishing in the non‐surgical treatment of peri ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC8360148/]
- 61. Do ultrasonic instrumentation and air polishing procedures ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12474538/]
- 62. Air Polishing Dangers: Case Study of an Injured Dental ... [https://www.todaysrdh.com/air-polishing-dangers-case-study-of-an-injured-dental-patient/]
- 63. An in vitro study exploring a new method for managing peri ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12246072/]
- 64. Factors Affecting Dental Implant Failure: A Retrospective ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12193482/]
- 65. World Academy of Sciences Journal [https://www.spandidos-publications.com/10.3892/wasj.2025.409]
- 66. Photodynamic therapy and peri-implant diseases [https://www.frontiersin.org/journals/oral-health/articles/10.3389/froh.2025.1614982/full]
- 67. Surveying coating strategies for peri-implantitis management [https://pubmed.ncbi.nlm.nih.gov/40689548/]
- 68. DENTAL IMPLANT FAILURE AND RETRIEVAL ... [https://www.frontiersin.org/journals/oral-health/articles/10.3389/froh.2025.1667808/full]
- 69. Dental Implants: Troubleshooting Guide For Dentists [https://avantdental.com.au/clinical/troubleshooting-guide-for-dental-implants/]
- 70. Prosthetic design and choice of components for ... [https://pubmed.ncbi.nlm.nih.gov/38789753/]
- 71. Can We Prevent Peri-Implantitis with an Adequate ... [https://blog.iti.org/clinical-insights/can-we-prevent-peri-implantitis-with-an-adequate-prosthetic-design/]
- 72. A Complete Guide to Caring for Your Dental Implants [https://www.barclayfamilydental.com/a-complete-guide-to-caring-for-your-dental-implants-single-tooth-all-on-x-and-full-mouth-solutions]
- 73. Dental Implant Maintenance and Lifespan [https://foreondental.com/blog/dental-implant-maintenance-and-lifespan/]
- 74. How to Care for Your Dental Implants: Key Tips for Longevity [https://gargdmd.com/blog/how-to-care-for-your-dental-implants/]
- 75. Stability of peri-implantitis surgical reconstructive therapy-a ... [https://pubmed.ncbi.nlm.nih.gov/38147180/]
- 76. What every dental practitioner should know about how to ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC10005927/]
- 77. Peri-Implantitis and How It Can Affect Your Dental Implants [https://lastingsmiles.com/peri-implantitis-and-how-it-can-affect-your-dental-implants/]
- 78. Comparison of Non-Surgical Methods for Implant Surface ... [https://www.mdpi.com/2077-0383/14/20/7244]
- 79. Comparative outcomes of endodontically treated teeth ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12239130/]
- 80. Clinical efficacy of dental implants placement without flap ... [http://www.jdir.org/journal/view.html?doi=10.54527/jdir.2025.44.3.31]
- 81. Dental Implant Statistics 2025-2024 & 2023 [https://impressionsdental.com/blog/dental-implant-statistics/]
- 82. What Is The Dental Implant Success Rate? Stats & Insights! [https://missionimplantcenter.com/dental-implant-success-rate-insights/]
- 83. Dental Implant Failures: Who's to Blame? [https://msdi-dental.com/2024/11/06/dental-implant-failures-analysis/]
- 84. Machine learning-based meta-analysis reveals gut ... [https://www.nature.com/articles/s41467-025-56829-3]
- 85. It takes guts to learn: machine learning techniques for ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC8786294/]
- 86. Profiling the Bacterial Microbiome Across Peri-Implant ... [https://pubmed.ncbi.nlm.nih.gov/40858525/]
- 87. How shotgun metagenomics can improve early diagnosis ... [https://www.efp.org/publications-hub/how-shotgun-metagenomics-can-improve-early-diagnosis-of-peri-implant-diseases/]
- 88. Transcriptomic and Metagenomic Biomarkers in Peri ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12452457/]
- 89. Cost-effectiveness and long-term outcomes of periodontal ... [https://pubmed.ncbi.nlm.nih.gov/41159906/]
- 90. Best oral self-care practices for peri-implant conditions and ... [https://www.frontiersin.org/journals/oral-health/articles/10.3389/froh.2025.1657025/full]
- 91. Contemporary oral health strategies: from tooth ... [https://www.straumann.com/en/discover/youtooth/article/implantology/2025/contemporary-oral-health-strategies-from-tooth-preservation-to-long-term-peri-implant-care.html]
- 92. Systematic Review Efficacy of supportive peri-implant ... [https://www.sciencedirect.com/science/article/pii/S0002817725004970]
- 93. The Future of Dental Implants: A Narrative Review ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12442331/]
- 94. A Guide to Biomarker Validation [https://sonraianalytics.com/a-guide-to-biomarker-validation/]
- 95. Ten quick tips for biomarker discovery and validation ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC9371329/]
- 96. Integrative microbiome- and metatranscriptome-based ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12381052/]
- 97. Screening, identification, and experimental validation of ... [https://hereditasjournal.biomedcentral.com/articles/10.1186/s41065-025-00525-1]
- 98. Diagnostic accuracy of bone-related biomarkers on peri ... [https://bmcoralhealth.biomedcentral.com/articles/10.1186/s12903-025-06578-y]
- 99. Navigating Biomarker Testing: Frequently Asked Questions [https://www.lung.org/lung-health-diseases/lung-disease-lookup/lung-cancer/symptoms-diagnosis/biomarker-testing/biomarker-faqs]
- 100. Diagnostic Significance of Peri-Implant Crevicular Fluid ... [https://ifnmujournal.com/gmj/article/view/e-GMJ2025-A20]
- 101. Quantitative Image Analysis for Tissue Biomarker Use [https://pmc.ncbi.nlm.nih.gov/articles/PMC8354563/]