Advanced Hydrogel Fabrication for Wound Healing: From Biomaterial Design to Clinical Translation
This article provides a comprehensive analysis of the latest advancements in hydrogel fabrication for wound healing applications, tailored for researchers and drug development professionals.
Advanced Hydrogel Fabrication for Wound Healing: From Biomaterial Design to Clinical Translation
Abstract
This article provides a comprehensive analysis of the latest advancements in hydrogel fabrication for wound healing applications, tailored for researchers and drug development professionals. It systematically explores the foundational principles of hydrogel design, including the critical properties of natural and synthetic polymers. The scope extends to cutting-edge fabrication methodologies, from 3D bioprinting to self-assembly, and their application in creating multifunctional and intelligent wound dressings. It further addresses key challenges in optimization, such as enhancing mechanical robustness and controlling drug release, and validates these approaches through a critical examination of preclinical and clinical evidence. By synthesizing insights across these four core intents, this review serves as a strategic guide for the continued development of clinically effective hydrogel-based therapies.
The Building Blocks of Healing: Hydrogel Fundamentals and Biomaterial Selection
The Physiology of Wound Healing and Rationale for Hydrogel Intervention
Chronic wounds, such as diabetic foot ulcers, venous ulcers, and pressure ulcers, represent a formidable global health challenge, affecting over 40 million patients annually and incurring healthcare costs exceeding $50 billion per year worldwide [1]. These wounds are characterized by a failure to proceed through an orderly and timely reparative process to produce anatomic and functional integrity [2]. The complex pathophysiology of chronic wounds includes persistent inflammation, elevated oxidative stress, bacterial colonization, biofilm formation, and impaired angiogenesis [1] [3]. Traditional wound dressings, including gauze and hydrocolloids, often fail to address this complex microenvironment, leading to prolonged healing times and increased risk of complications [4]. In contrast, hydrogel-based dressings have emerged as a promising class of biomaterials that actively support the healing process by maintaining a moist environment, providing a protective barrier, and delivering therapeutic agents [4] [5]. This application note examines the physiological basis of wound healing and establishes the scientific rationale for hydrogel intervention, providing researchers with detailed protocols for evaluating hydrogel efficacy in wound healing applications.
The Physiology of Wound Healing
Wound healing is a complex, dynamic process that restores function and integrity to damaged tissue. This process traditionally unfolds through four overlapping, precisely regulated phases: hemostasis, inflammation, proliferation, and remodeling [2] [1].
Phases of Normal Wound Healing
Hemostasis: Immediately following injury, vasoconstriction occurs to reduce blood loss, followed by platelet aggregation at the site of vessel injury. These activated platelets form a provisional clot and release growth factors and chemokines that initiate the subsequent inflammatory phase [2] [1]. Platelets simultaneously release growth factors and recruit immune cells, establishing the foundation for tissue repair [6].
Inflammation: Characterized by the sequential infiltration of neutrophils and macrophages. Neutrophils are the first responders, clearing pathogens and debris through phagocytosis and releasing reactive oxygen species (ROS) [6]. Macrophages then replace neutrophils, transforming from a pro-inflammatory (M1) to an anti-inflammatory (M2) phenotype, which is crucial for resolving inflammation and initiating tissue repair [6] [1]. Dysregulation in this phase is a hallmark of chronic wounds.
Proliferation: This phase involves re-epithelialization, angiogenesis, and collagen synthesis. Fibroblasts migrate into the wound bed and produce extracellular matrix (ECM) components, particularly type III collagen. Simultaneously, new blood vessels form to restore oxygen and nutrient supply to the healing tissue [6] [2].
Remodeling: The final phase can last for months to years, during which fragile type III collagen is gradually replaced and reorganized into stronger type I collagen, providing mechanical robustness to the repaired tissue [6]. This process determines the ultimate strength and appearance of the healed wound, with excessive ECM deposition leading to fibrotic scarring [1] [3].
The Chronic Wound Microenvironment
Chronic wounds are characterized by a pathological deviation from the normal healing sequence, often stalling in the inflammatory phase due to a complex interplay of factors [1]. Key characteristics of the chronic wound microenvironment include:
- Persistent Inflammation: Sustained elevated levels of pro-inflammatory cytokines (e.g., IL-6, TNF-α) and continuous neutrophil activity create a destructive cycle of inflammation and tissue damage [1].
- Elevated Oxidative Stress: Excessive accumulation of reactive oxygen species (ROS) at the wound site creates a vicious ROS-inflammation cycle that heavily hinders tissue regeneration [3].
- Bacterial Biofilms: Drug-resistant bacterial biofilms, particularly in diabetic foot ulcers, increase drug resistance and aggravate inflammatory responses, significantly impeding healing [3].
- Impaired Angiogenesis: Inadequate formation of new blood vessels results in insufficient blood supply with limited oxygen and nutrient delivery to the wound bed [3].
Table 1: Key Biomarkers in the Chronic Wound Microenvironment
| Biomarker Category | Specific Markers | Significance in Chronic Wounds |
|---|---|---|
| Physiological Parameters | Temperature, Oxygen levels, Humidity | Elevated temperature indicates inflammation; hypoxia indicates impaired perfusion [6] |
| Biochemical Parameters | pH, Glucose, Uric acid | Acidic pH may indicate infection; hyperglycemia suggests diabetic dysregulation [6] |
| Inflammatory Cytokines | IL-6, TNF-α, IL-10 | Persistent elevation of pro-inflammatory cytokines indicates chronic inflammation [6] [1] |
| Oxidative Stress Markers | Reactive Oxygen Species (ROS) | Excessive ROS creates oxidative stress-inflammation cycle [3] |
| Enzymatic Activity | Matrix Metalloproteinases (MMPs) | Overexpression leads to excessive ECM degradation [1] |
Rationale for Hydrogel Intervention
Hydrogels are three-dimensional, hydrophilic polymeric networks with high water content that closely mimic the native extracellular matrix (ECM), making them ideal biomaterials for wound management [7] [5]. Their unique properties directly address the pathophysiological challenges present in chronic wounds.
Fundamental Advantages of Hydrogels
Moist Wound Environment: Hydrogels maintain a moist wound environment, which has been clinically proven to accelerate epithelialization and promote granulation tissue formation compared to dry wound beds [4]. Their high water content (often exceeding 90%) prevents wound desiccation while absorbing excess exudate [4] [5].
Gas Permeability: The porous structure of hydrogels allows for oxygen permeation to the wound bed while providing a physical barrier against external pathogens [5].
Biocompatibility and Biodegradability: Hydrogels can be fabricated from natural polymers such as chitosan, hyaluronic acid, alginate, and collagen, which exhibit inherent biocompatibility, low immunogenicity, and tunable degradation profiles [7] [5].
Thermal Insulation and Pain Relief: The high water content provides cooling sensation and pain relief through nerve ending insulation, significantly improving patient comfort during dressing changes [4].
Advanced Functional Capabilities
Beyond these fundamental advantages, advanced hydrogel systems offer sophisticated therapeutic capabilities:
Self-Healing Properties: Incorporating dynamic covalent bonds (e.g., Schiff base, disulfide bonds) or non-covalent interactions (e.g., hydrogen bonding, host-guest interactions) enables hydrogels to autonomously repair damage after mechanical stress, restoring their structural integrity and extending their functional lifespan [7] [1]. This is particularly valuable for joint areas or wounds subject to movement.
Stimuli-Responsive Drug Delivery: Smart hydrogels can be engineered to release therapeutic agents in response to specific wound microenvironment triggers such as pH, temperature, enzyme activity, or ROS levels [7] [6]. For instance, Schiff base-crosslinked hydrogels degrade faster in the acidic environment of infected wounds, enabling targeted drug release [3].
Multifunctionality: Modern hydrogels can be designed with integrated properties including antibacterial activity (through incorporation of silver nanoparticles, antimicrobial peptides), antioxidant capacity (via ceria nanozymes, gallic acid), pro-angiogenic effects (through growth factor delivery), and even neural regeneration capabilities [1] [3].
Phase-Adaptive Regulation: Recent innovations include hydrogels with phase-adaptive regulating functions that provide different therapeutic actions according to the specific stage of wound healing. For example, a dynamically Schiff base-crosslinked hydrogel (F/R gel) can first eliminate multidrug-resistant bacterial biofilms, then interrupt the oxidative stress-inflammation cycle, and subsequently promote angiogenesis while suppressing fibrotic scarring [3].
Table 2: Hydrogel Functionalization Strategies for Chronic Wound Management
| Functionalization Approach | Mechanism of Action | Representative Agents |
|---|---|---|
| Antimicrobial Integration | Disrupt bacterial cell membranes, prevent biofilm formation | Silver nanoparticles, ε-polylysine, antimicrobial peptides [1] [3] |
| Antioxidant Incorporation | Scavenge excess ROS, break ROS-inflammation cycle | Ceria nanozymes, gallic acid, polyphenols [1] [3] |
| Pro-angiogenic Enhancement | Stimulate new blood vessel formation | Basic fibroblast growth factor (bFGF), VEGF [6] [3] |
| Immunomodulation | Shift macrophages from M1 to M2 phenotype | IL-10, TGF-β, specialized nanoparticles [1] |
| Conductive Properties | Enable real-time wound monitoring | MXene, polypyrrole, PEDOT:PSS [6] [1] |
| Scar Suppression | Modulate fibroblast activity to prevent fibrosis | c-Jun siRNA, TGF-β inhibitors [3] |
Experimental Protocols for Hydrogel Evaluation
Protocol 1: Fabrication of a Dynamic Schiff Base-Crosslinked Hydrogel
This protocol describes the synthesis of an injectable, self-healing hydrogel through Schiff base formation between ε-polylysine (εPL) and aldehyde-modified hyaluronic acid (HA-CHO), based on methodology from a recent groundbreaking study [3].
Research Reagent Solutions:
- Aldehyde-Modified Hyaluronic Acid (HA-CHO): Synthesized by oxidizing hyaluronic acid with sodium periodate. Serves as the primary polymer backbone with aldehyde functional groups for crosslinking.
- ε-Polylysine (εPL): Cationic antimicrobial polypeptide containing primary amino groups that react with aldehyde groups to form Schiff base bonds.
- εPL-Modified Oxygen-Deficient Nanoceria (εPL-CeOv Nanozyme): Cerium oxide nanoparticles with surface-modified εPL, providing ROS-scavenging capability and antimicrobial activity.
- Drug-Loaded PLGA Microcapsules: Poly(lactic-co-glycolic acid) microspheres containing therapeutic agents (e.g., bFGF and c-Jun siRNA) for sustained release.
Procedure:
- Polymer Solution Preparation: Dissolve HA-CHO in PBS (pH 7.4) at a concentration of 4% (w/v). Separately, dissolve εPL in PBS at 5% (w/v).
- Nanozyme Incorporation: Uniformly disperse εPL-CeOv nanozyme (2.8 ± 0.8 nm) in the εPL solution at a concentration of 1 mg/mL using gentle sonication.
- Microcapsule Integration: Suspend F/R MCs (loaded with bFGF and c-Jun siRNA) in the HA-CHO solution at a concentration of 2% (w/v).
- Gelation Process: Mix the two component solutions (HA-CHO with MCs and εPL with nanozyme) in a 1:1 volume ratio. Rapid Schiff base formation will occur within approximately 3 seconds, forming a stable three-dimensional hydrogel network.
- Characterization: Confirm successful gelation through rheological measurements, showing storage modulus (G') exceeding loss modulus (G") with Young's modulus approximately 6.69 kPa.
Quality Control:
- Assess shear-thinning behavior by monitoring G' and G" under increasing shear strain (0%-1000% at 1 Hz).
- Evaluate self-healing properties through strain amplitude alternation tests (cycling between 1% and 500% strain).
- Verify porous microstructure using scanning electron microscopy (SEM).
Protocol 2: In Vitro Evaluation of Hydrogel Properties
Swelling and Degradation Kinetics:
- Cut hydrogel samples into standardized discs (e.g., 10 mm diameter, 2 mm thickness).
- Measure initial dry weight (Wd), then immerse in PBS (pH 7.4) or simulated wound fluid (pH 5.5-6.5).
- At predetermined time points, remove samples, gently blot excess surface liquid, and record wet weight (Ww).
- Calculate swelling ratio as (Ww - Wd)/Wd.
- For degradation, monitor mass loss over time and record complete dissolution time.
Drug Release Profiling:
- Prepare hydrogel samples incorporating fluorescently labeled therapeutic agents.
- Immerse in release medium under sink conditions at 37°C with gentle agitation.
- At designated intervals, collect and replace release medium.
- Analyze collected samples using fluorescence spectroscopy or HPLC to determine cumulative drug release.
- Compare release kinetics between physiological (pH 7.4) and acidic (pH 5.5-6.5) conditions to confirm pH-responsive behavior.
Antimicrobial Efficacy Testing:
- Prepare bacterial suspensions of clinically relevant strains (e.g., Staphylococcus aureus, Pseudomonas aeruginosa, methicillin-resistant S. aureus [MRSA]) in nutrient broth.
- Incubate hydrogel discs with bacterial suspensions at 37°C for 24 hours.
- Determine minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) using standard microdilution methods.
- Assess biofilm inhibition by measuring biomass formation using crystal violet staining.
Antioxidant Activity Assessment:
- Treat cells (e.g., fibroblasts, macrophages) with hydrogen peroxide or lipopolysaccharide to induce oxidative stress.
- Apply hydrogel extracts or directly culture cells on hydrogel surfaces.
- Measure intracellular ROS levels using DCFH-DA fluorescence probe.
- Quantify expression of antioxidant enzymes (SOD, CAT, GPx) using ELISA or Western blot.
Protocol 3: In Vivo Assessment in Diabetic Wound Models
Animal Model Preparation:
- Use 8-10 week old db/db mice or streptozotocin-induced diabetic C57BL/6 mice.
- Anesthetize animals and create full-thickness excisional wounds (6-8 mm diameter) on the dorsal skin.
- Infect wounds with MRSA or P. aeruginosa (1×10^8 CFU) for infected wound models.
Treatment Groups:
- Group 1: Untreated control
- Group 2: Conventional dressing (e.g., gauze)
- Group 3: Basic hydrogel dressing
- Group 4: Advanced multifunctional hydrogel (e.g., F/R gel)
Wound Monitoring and Analysis:
- Macroscopic Assessment: Photograph wounds daily and calculate wound area reduction percentage.
- Histological Analysis: At days 7, 14, and 21 post-wounding, harvest wound tissues for:
- H&E staining to assess re-epithelialization and granulation tissue formation
- Masson's trichrome staining to evaluate collagen deposition and organization
- Immunofluorescence staining for CD31 (angiogenesis), F4/80 (macrophage infiltration), and α-SMA (myofibroblasts)
- Molecular Analysis:
- Measure inflammatory cytokine levels (TNF-α, IL-6, IL-1β, IL-10) using ELISA
- Assess oxidative stress markers (SOD, MDA, MPO) in wound tissues
- Evaluate expression of fibrotic markers (TGF-β, α-SMA, collagen I/III) using RT-qPCR or Western blot
- Scar Assessment: At day 28, evaluate scar formation using:
- Visual analog scales
- Histological scoring of collagen architecture
- Measurement of scar elevation index
Visualization of Wound Healing and Hydrogel Action Mechanisms
Diagram 1: Wound Healing Physiology and Hydrogel Intervention Points. This diagram illustrates the sequential phases of normal wound healing, the pathophysiological deviations in chronic wounds, and the multiple intervention points where advanced hydrogel dressings exert their therapeutic effects.
The physiological process of wound healing represents an intricate cascade of cellular and molecular events that, when dysregulated, leads to chronic, non-healing wounds. Hydrogel-based interventions provide a sophisticated, multifaceted approach to addressing the complex pathophysiology of these challenging wounds. Through their unique capacity to maintain a moist wound environment, provide structural support, deliver therapeutic agents in a spatiotemporally controlled manner, and dynamically respond to the wound microenvironment, hydrogels represent a paradigm shift in wound management. The experimental protocols outlined in this application note provide researchers with robust methodologies for developing and evaluating next-generation hydrogel dressings, with the ultimate goal of restoring timely, anatomically functional, and scar-free tissue repair for patients suffering from chronic wounds. As hydrogel technology continues to advance, incorporating increasingly sophisticated functionalities such as real-time monitoring, closed-loop feedback systems, and personalized therapeutic regimens, these biomaterials are poised to revolutionize wound care and significantly improve patient outcomes.
The management of acute and chronic wounds presents a significant global healthcare challenge. Chronic wounds, such as diabetic foot ulcers, venous leg ulcers, and pressure ulcers, are characterized by a failure to proceed through an orderly and timely healing process, often stalling in the inflammatory phase due to persistent bacterial infection, prolonged inflammation, impaired angiogenesis, and elevated levels of matrix metalloproteinases (MMPs) [8] [9]. Conventional wound dressings, including gauze, often prove inadequate as they can adhere to the wound bed, cause secondary injury upon removal, and fail to provide an optimal healing environment [9].
In response to these limitations, hydrogel-based wound dressings have emerged as a promising advanced therapeutic strategy. Hydrogels are three-dimensional, hydrophilic polymer networks that can absorb large amounts of water while maintaining their structure, thereby providing a moist wound environment conducive to healing [10] [11]. Among them, hydrogels fabricated from natural polymers—particularly chitosan, hyaluronic acid, alginate, and collagen—offer distinct advantages due to their inherent biocompatibility, biodegradability, low immunogenicity, and bioactivity [8] [11]. These materials closely mimic the native extracellular matrix (ECM), facilitating cell adhesion, proliferation, and migration, and can be engineered as delivery platforms for therapeutic agents like antimicrobials, nanoparticles, growth factors, and exosomes [8]. This application note details the bioactive properties of these four key natural polymers and provides standardized protocols for their incorporation into hydrogels for wound healing applications, framed within a broader thesis on advanced hydrogel fabrication.
Bioactive Properties and Mechanisms of Action
The efficacy of natural polymer-based hydrogels in wound healing stems from their diverse and synergistic bioactive properties, which actively modulate the wound microenvironment to promote regeneration.
Table 1: Bioactive Properties of Natural Polymers in Wound Healing
| Polymer | Source | Key Bioactive Properties | Role in Wound Healing |
|---|---|---|---|
| Chitosan | Crustacean exoskeletons, insects [12] | Antibacterial (cationic nature disrupts bacterial membranes) [12], Hemostatic [13], promotes granulation tissue formation [14] | Controls infection, accelerates blood clotting, supports new tissue growth |
| Hyaluronic Acid (HA) | Bacterial fermentation, animal tissues [15] | Angiogenic, Anti-inflammatory, regulates collagen deposition [15] [9] | Promotes blood vessel formation, modulates inflammation, improves tissue remodeling |
| Alginate | Seaweed [16] [17] | High absorbency, forms gel in contact with exudate, facilitates ion exchange (Ca²âº/Naâº) [16] [17] | Manages wound exudate, maintains moist environment, supports debridement |
| Collagen | Bovine, porcine, marine tissues [10] | Cell adhesion & migration, low antigenicity, promotes fibroblast proliferation [10] | Serves as a scaffold for cellular infiltration, fundamental for all healing stages |
The wound healing process is a complex cascade that can be disrupted in chronic states. The following diagram illustrates the normal healing pathway and how chronic wounds deviate from it, highlighting the therapeutic targets for natural polymer hydrogels.
Diagram 1: The wound healing cascade and points of failure in chronic wounds. Chronic wounds often stall in the inflammatory phase due to a combination of disruptive factors, preventing progression to proliferation and remodeling [8] [9] [11].
Application Notes and Experimental Protocols
Chitosan-Based Thermosensitive Hydrogel
Application Notes: Thermosensitive chitosan hydrogels are injectable systems that exist as liquids at room temperature and undergo a sol-gel transition at body temperature (37°C). This allows for minimally invasive application that perfectly conforms to irregular wound beds [12]. A common formulation involves combining chitosan with sodium glycerophosphate (GP).
Protocol: Fabrication of Chitosan-Sodium Glycerophosphate (CS-GP) Hydrogel [12]
Objective: To prepare an injectable, thermosensitive hydrogel for drug delivery and wound dressing.
Materials:
- Chitosan (medium molecular weight, deacetylation degree > 85%)
- Sodium β-glycerophosphate (GP)
- Hydrochloric acid (HCl, 0.1 M)
- Deionized water
- Magnetic stirrer with heating plate
- Ice bath
- pH meter
Procedure:
- Chitosan Solution Preparation: Dissolve 2 g of chitosan in 100 mL of 0.1 M HCl solution under vigorous stirring at room temperature overnight to obtain a clear, viscous 2% (w/v) chitosan solution.
- Cooling: Cool the chitosan solution to 4°C in an ice bath.
- GP Solution Preparation: Prepare a 50% (w/v) aqueous solution of GP and cool it to 4°C.
- Mixing: Slowly add the chilled GP solution dropwise into the chilled chitosan solution under constant stirring. Maintain the temperature below 5°C during this process to prevent premature gelation.
- pH Adjustment: Continue stirring until a homogeneous solution is formed. The final pH should be between 7.0 and 7.2; adjust with dilute NaOH or HCl if necessary.
- Gelation Test: Incubate a small aliquot of the final mixture in a water bath at 37°C. The gelation time is typically between 2 to 10 minutes, forming an opaque gel.
The mechanism behind the sol-gel transition is a temperature-driven shift in molecular interactions, as shown below.
Diagram 2: The sol-gel transition mechanism in LCST-type thermosensitive hydrogels like CS-GP. The shift from sol to gel is driven by a change in the dominant molecular interaction from hydrophilic to hydrophobic as temperature increases, which is reflected in the system's Gibbs free energy (ΔG = ΔH - TΔS) [12].
Hyaluronic Acid-Based Multifunctional Hydrogel
Application Notes: Hyaluronic acid (HA) is a core component of the ECM and is crucial for regulating inflammation and promoting tissue regeneration. Methacrylated HA (HAMA) can be crosslinked to form hydrogels with tunable mechanical properties and high stability, suitable for loading and sustaining the release of therapeutic agents [15] [9].
Protocol: Fabrication of an HA-Based Hybrid (HMGF) Hydrogel [15]
Objective: To synthesize a photocrosslinked HA hydrogel synergized with glycyrrhizic acid (GA) and Fe³⺠for antibacterial and anti-inflammatory activity.
Materials:
- Methacrylated Hyaluronic Acid (HAMA)
- Acrylamide (AM)
- Glycyrrhizic acid (GA)
- Iron (III) chloride (FeCl₃)
- Photoinitiator (e.g., Irgacure 2959)
- UV light source (e.g., 365 nm wavelength)
Procedure:
- Polymer Solution Preparation: Dissolve HAMA and AM in PBS or deionized water to a final concentration of 3-5% (w/v).
- Functionalization: Add GA (e.g., 1-2 mg/mL) and FeCl₃ (e.g., 0.5-1 mM) to the polymer solution. Stir thoroughly to ensure a homogeneous mixture.
- Photoinitiator Addition: Add the photoinitiator Irgacure 2959 to a final concentration of 0.05-0.1% (w/v) and stir until completely dissolved.
- Molding and Crosslinking: Pour the solution into a mold matching the wound shape. Expose the mold to UV light (365 nm, 5-10 mW/cm²) for 3-10 minutes to initiate free radical polymerization and form a stable, crosslinked hydrogel.
- Sterilization and Storage: The resulting HMGF hydrogel can be sterilized under UV light and should be stored at 4°C in a sealed container until use.
Alginate-Based Hydrogel for Sustained Drug Delivery
Application Notes: Alginate hydrogels are ideal for exuding wounds due to their high absorbency. Ionically crosslinked alginate gels can be used for the sustained release of antimicrobial peptides (AMPs) to combat biofilm-associated infections in chronic wounds like diabetic foot ulcers [16].
Protocol: Fabrication of an Alginate-Based Antimicrobial Peptide (AMP) Delivery Hydrogel [16]
Objective: To create an alginate hydrogel for the sustained release of an antimicrobial peptide.
Materials:
- Sodium Alginate (high G-content preferred)
- Antimicrobial Peptide (AMP)
- Calcium Chloride (CaClâ‚‚) solution (e.g., 50-100 mM)
- Deionized water
Procedure:
- Alginate/AMP Solution: Dissolve sodium alginate (e.g., 2% w/v) and the selected AMP (e.g., 0.1-1 mg/mL) in deionized water under gentle stirring to avoid peptide denaturation.
- Gel Formation via Ionic Crosslinking:
- Method A (In-situ gelation): Mix the alginate/AMP solution with a soluble calcium salt (e.g., CaCO₃) and a slow acidifier (e.g., glucono-δ-lactone, GDL) to gradually release Ca²⺠ions and form a homogeneous gel.
- Method B (Diffusion gelation): Add the alginate/AMP solution dropwise into a bath of gently stirred CaClâ‚‚ solution. The droplets will instantaneously form hydrogel beads. Alternatively, pour the solution into a mold and submerge it in the CaClâ‚‚ solution for bulk gel formation.
- Washing and Equilibration: After gelation (typically 30-60 minutes), remove the hydrogel, rinse with deionized water to remove unreacted ions and surface-bound AMP, and equilibrate in a buffer (e.g., PBS, pH 7.4) before use or characterization.
Collagen-Based Bioactive Hydrogel
Application Notes: Collagen hydrogels provide an excellent biomimetic scaffold that supports all phases of wound healing. Their main limitations—mechanical strength and rapid degradation—can be improved through crosslinking or forming composite hydrogels with other polymers like chitosan or alginate [10].
Protocol: Fabrication of a Crosslinked Collagen-Chitosan Composite Hydrogel [10]
Objective: To prepare a mechanically stable collagen-based composite hydrogel with enhanced properties for wound dressing.
Materials:
- Type I Collagen solution (e.g., from bovine or rat tail tendon)
- Chitosan
- Acetic acid (0.1 M)
- Crosslinker (e.g., PEGDE 500 or Genipin)
- Deionized water
Procedure:
- Chitosan Solution: Dissolve chitosan in 0.1 M acetic acid to obtain a 1-2% (w/v) solution.
- Mixing: Gently mix the collagen solution (e.g., 3-5 mg/mL) and the chitosan solution in a desired mass ratio (e.g., 1:1 or 2:1 collagen:chitosan) under slow stirring at 4°C to prevent collagen fibrillation.
- pH Neutralization: Add a neutralization medium (e.g., NaOH or sodium bicarbonate solution) to raise the pH to approximately 7.4, inducing the initial physical gelation of collagen.
- Chemical Crosslinking: Add a chemical crosslinker like PEGDE 500 (e.g., 1-5% w/w of polymer) to the blend. Incubate the mixture at 37°C for 2-24 hours to form a covalently crosslinked, stable composite hydrogel.
- Hydration and Storage: Wash the formed hydrogel with PBS to remove residual crosslinker and maintain it in a hydrated state at 4°C.
Table 2: Quantitative Performance Data of Natural Polymer Hydrogels
| Hydrogel System | Key Performance Metrics | Experimental Results | Reference Model |
|---|---|---|---|
| Chitosan Thermosensitive | Gelation Time at 37°C | 2 - 10 minutes | Chitosan-GP Hydrogel [12] |
| HA-Based Hybrid (HMGF) | Wound Closure Rate (in vivo, Day 14) | ~90% closure | HMGF Hydrogel [15] |
| Alginate for Drug Delivery | Swelling Capacity (PBS) | Up to 90 g/dm² | Gelatin-Alginate Hydrogel [17] |
| Collagen-Based | Water Vapor Transmission Rate (WVTR) | ~2750 g/m²/day | Collagen-Chitosan Hydrogel [10] |
| General Hydrogel | Antibacterial Efficacy (against S. aureus/E. coli) | Significant inhibition zone | PM@CS Hydrogel [13] |
The Scientist's Toolkit: Essential Research Reagents
Table 3: Key Reagent Solutions for Hydrogel Fabrication and Characterization
| Reagent / Material | Function / Application | Example Use Case |
|---|---|---|
| Methacrylated Polymers (e.g., HAMA) | Enables photocrosslinking for tunable mechanical properties and in-situ gelation. | Fabrication of structurally stable HA hydrogels [15]. |
| Sodium Glycerophosphate (GP) | A key component for inducing thermosensitivity in chitosan solutions. | Preparing injectable CS-GP hydrogels [12]. |
| Ionic Crosslinkers (e.g., CaClâ‚‚) | Induces rapid gelation of anionic polymers like alginate via ionic bridging. | Forming alginate beads or bulk gels for drug delivery [16] [17]. |
| Polyethylene Glycol Diglycidyl Ether (PEGDE) | A biocompatible chemical crosslinker for enhancing mechanical strength and stability. | Crosslinking gelatin-alginate [17] or collagen-chitosan [10] hydrogels. |
| Glycyrrhizic Acid & Fe³⺠Ions | Provides synergistic antibacterial and anti-inflammatory activity. | Functionalizing HA hydrogels for infected wound management [15]. |
| Cell-Free Probiotic Metabolites (CFPM) | Source of bioactive compounds (organic acids, bacteriocins) for antimicrobial activity. | Loading into chitosan hydrogels to create probiotic metabolite-based dressings [13]. |
| DBCO-Val-Cit-PABC-PNP | DBCO-Val-Cit-PABC-PNP, MF:C46H49N7O10, MW:859.9 g/mol | Chemical Reagent |
| Aminooxy-amido-PEG4-propargyl | Aminooxy-amido-PEG4-propargyl, MF:C13H24N2O6, MW:304.34 g/mol | Chemical Reagent |
The effective management of acute and chronic wounds remains a significant challenge in healthcare, driving the need for advanced therapeutic solutions. Hydrogels, three-dimensional hydrophilic polymer networks, have emerged as a cornerstone of modern wound care due to their ability to maintain a moist wound environment, absorb exudate, and facilitate autolytic debridement [18]. Among the various materials used in hydrogel fabrication, synthetic polymers—particularly polyvinyl alcohol (PVA), polyethylene glycol (PEG), and polyacrylamide (PAAm)—offer distinct advantages for wound healing applications, including precise tunability of physical properties, consistent quality, and controllable biodegradation profiles [19] [20]. These polymers can be engineered to create ideal wound dressings that protect against external contaminants, promote cell migration, and minimize interference with the natural healing process [21].
The versatility of PVA, PEG, and PAAm stems from their modifiable chemical structures, which enable researchers to fine-tune mechanical strength, swelling behavior, and bioadhesion properties to address specific clinical requirements. This application note explores the unique characteristics of these synthetic polymers, provides detailed experimental protocols for hydrogel fabrication, and presents quantitative data on their performance in wound healing applications, specifically within the context of a broader thesis on advanced hydrogel fabrication for improved wound management.
Polymer Properties and Tunability
The effectiveness of synthetic polymer-based hydrogels in wound healing applications derives from their customizable physical and chemical properties. The table below summarizes the key characteristics and tuning parameters for PVA, PEG, and PAAm:
Table 1: Tunable Properties of Synthetic Polymers for Wound Healing Hydrogels
| Polymer | Key Properties | Tunable Parameters | Cross-linking Methods | Wound Healing Advantages |
|---|---|---|---|---|
| PVA | Excellent mechanical strength, high biocompatibility, good water content [19] [22] | Molecular weight, degree of hydrolysis, concentration [23] | Freeze-thaw cycles, chemical cross-linkers (e.g., glutaraldehyde), salting-out [19] [22] | Creates robust, flexible dressings; promotes moist environment [23] |
| PEG | High hydrophilicity, biocompatibility, non-immunogenicity [20] | Molecular weight, branching density, functional end-groups | Physical entanglement, chemical cross-linking (e.g., with acrylamide) [20] | Enhances hydration; can be copolymerized for improved drug delivery [20] |
| PAAm | Responsive swelling behavior, functionalizable backbone | Co-monomer composition, cross-linking density | Free radical polymerization, covalent cross-linking [20] | Provides structural framework; enables controlled release of therapeutic agents [20] |
The mechanical and swelling properties of these polymers can be precisely controlled through synthetic parameters. For PVA, higher molecular weights (e.g., Mowiol 56–98 with Mw~195,000) and full hydrolysis (98.0–98.8%) produce cryogels with enhanced structural integrity, while partially hydrolyzed grades offer improved water absorption [23]. The freeze-thaw method, employing temperatures as low as -80°C, creates more open, interconnected structures with superior mechanical strength and elasticity compared to conventional -25°C freezing [23]. Incorporating PEG and PAAm into PVA-based systems further enhances functionality; for instance, PVA-co-AAm hydrogels demonstrate improved breaking strength, deformability, and compatibility with cutaneous tissue [20].
Quantitative Performance Data
Recent studies have provided quantitative evidence supporting the efficacy of synthetic polymer hydrogels in wound healing applications. The following table summarizes key experimental findings:
Table 2: Performance Metrics of Synthetic Polymer Hydrogels in Wound Healing Models
| Hydrogel Composition | Experimental Model | Key Performance Metrics | Results | Reference |
|---|---|---|---|---|
| PVA-based micro-patterned (checks pattern) | SD rat skin wounds | Wound Closure Rate (WCR) at day 7 | 90.4% WCR [21] | |
| PVA-based micro-patterned (checks pattern) | SD rat skin wounds | Time constant (Ï„) to reach 63.2% WCR | 2.7 days [21] | |
| PVA-co-AAm with bromelain | In vitro release study | Bromelain release profile | Sustained release over extended period [20] | |
| PVA/PEG/CuO nanocomposite (1% CuO) | Antimicrobial testing | Antibacterial activity against S. aureus and E. coli | Highest antibacterial properties [24] | |
| PVA/PEG/CuO nanocomposite | Cytocompatibility testing | Cell viability | >70% cell viability [24] | |
| PVA/CMC/PEG bi-layer | Full-thickness skin defects | Wound closure acceleration | Significant acceleration vs. controls [25] | |
| 8% PVA56-98 with 10% PG | Mechanical testing | Stretchability, durability, low adhesion | Optimal balance for wound dressing [23] |
The performance advantages of specific hydrogel designs are particularly notable. Micro-patterned hydrogels with checks patterns demonstrated significantly superior wound healing efficacy compared to wave, line, and non-patterned hydrogels, achieving a 90.4% wound closure rate within 7 days compared to 65.1% in the vehicle control group [21]. This enhanced performance is attributed to increased surface area and volume in the vertical direction, which positively influences cellular responses and wound fluid management [21].
Experimental Protocols
Fabrication of PVA-based Cryogels via Freeze-Thaw Method
Materials: Polyvinyl alcohol (e.g., Mowiol 56–98, Mw~195,000; DH = 98.0–98.8%), propylene glycol, distilled water, sodium chloride, mucin (from porcine stomach, type II) [23].
Procedure:
- Prepare an 8% (w/w) PVA solution by dissolving PVA in distilled water at 90°C with slow stirring (1.5-4 hours until fully dissolved)
- Add 10% (w/w) propylene glycol as a plasticizer and mix thoroughly
- Degas the solution to remove air bubbles
- Pour the solution into appropriate molds for membrane formation
- Subject the samples to freezing at -80°C for 12-24 hours, followed by thawing at room temperature for 8-12 hours
- Repeat the freeze-thaw cycle 3-6 times to increase crystallinity and mechanical strength
- Characterize the resulting cryogels for mechanical properties, absorption capacity, and microstructure [23]
Note: The number of freeze-thaw cycles significantly impacts the final material properties. Higher cycles (up to 6) increase crystallinity, toughness, and tensile properties while decreasing the swelling coefficient [22].
Synthesis of PVA-co-AAm and PEG-co-AAm Hydrogels for Drug Delivery
Materials: PVA, PEG, acrylamide (AAm), N,N'-methylene-bis-acrylamide (BIS), ammonium persulfate (APS), N,N,N',N'-tetramethylethylenediamine (TEMED), bromelain [20].
Procedure:
- Prepare separate solutions of PVA and PEG in distilled water
- Dissolve AAm and BIS (cross-linker) in the polymer solutions
- Add APS (initiator) and TEMED (catalyst) to initiate copolymerization
- For drug-loaded hydrogels, incorporate bromelain (or other therapeutic agents) into the solution prior to gelation
- Plate the solution and allow copolymerization to occur (typically rapid)
- Characterize swelling capacity by immersing hydrogels in solution and measuring weight gain at intervals (up to 24 hours)
- Evaluate mechanical properties through tensile testing and deformability measurements [20]
Applications: These copolymer hydrogels are particularly suitable for controlled release of therapeutic proteins like bromelain, which demonstrates anti-inflammatory and debridement properties beneficial for wound healing [20].
Preparation of PVA/CMC/PEG Bi-Layer Hydrogels with Gradient Pore Sizes
Materials: PVA, carboxymethyl cellulose (CMC), PEG [25].
Procedure:
- Prepare single-layer PVA/CMC/PEG hydrogels using a thawing-freezing method
- Control pore size through processing parameters
- Fabricate bi-layer hydrogels with gradually increasing pore sizes from upper to lower layer
- Ensure strong bonding between the two layers
- Characterize physical properties, including bacterial penetration resistance and moisture retention capability
- Evaluate wound healing efficacy using full-thickness skin defect models [25]
Advantages: The bi-layer design with gradient pore sizes provides dual functionality—the denser upper layer protects against bacterial penetration while the more porous lower layer facilitates fluid management and tissue integration [25].
Visualization of Experimental Workflows
Hydrogel Fabrication and Evaluation Pathway
Wound Healing Mechanism of Action
Research Reagent Solutions
Table 3: Essential Research Reagents for Synthetic Polymer Hydrogel Fabrication
| Reagent/Category | Specific Examples | Function | Application Notes |
|---|---|---|---|
| Base Polymers | PVA (Mowiol series: 8-88, 56-98), PEG (various MW), Acrylamide | Primary matrix formation | Select PVA based on MW and hydrolysis degree; Higher MW (e.g., 56-98) for mechanical strength [23] |
| Cross-linking Agents | Glutaraldehyde, N,N'-methylene-bis-acrylamide (BIS), Ammonium persulfate (APS) | Create 3D network structure | Chemical cross-linkers enhance stability; Physical cross-linking improves biocompatibility [19] [20] |
| Plasticizers | Propylene glycol, Glycerol | Enhance flexibility and stretchability | 10% (w/w) PG optimizes mechanical properties [23] |
| Active Compounds | Bromelain, Neomycin, Copper oxide nanoparticles | Provide therapeutic activity | Bromelain offers anti-inflammatory and debriding action; CuO adds antimicrobial properties [20] [26] [24] |
| Characterization Reagents | Mucin, Bradford reagent, Azocasein | Assess functional performance | Evaluate swelling, protein content, and enzymatic activity [20] [23] |
Synthetic polymers PVA, PEG, and PAAm provide an exceptionally versatile platform for developing advanced wound healing hydrogels with tunable properties. Through controlled fabrication techniques such as freeze-thaw cycling, chemical cross-linking, and copolymerization, researchers can precisely engineer hydrogels with optimal mechanical strength, swelling behavior, and biofunctional characteristics. The quantitative data presented demonstrates the significant potential of these materials to accelerate wound closure, enhance collagen expression, and prevent infection. As research progresses, the integration of innovative elements such as micro-patterning, nanocomposites, and bi-layer designs will further expand the capabilities of synthetic polymer hydrogels, ultimately leading to more effective wound management solutions that address the complex challenges of both acute and chronic wounds.
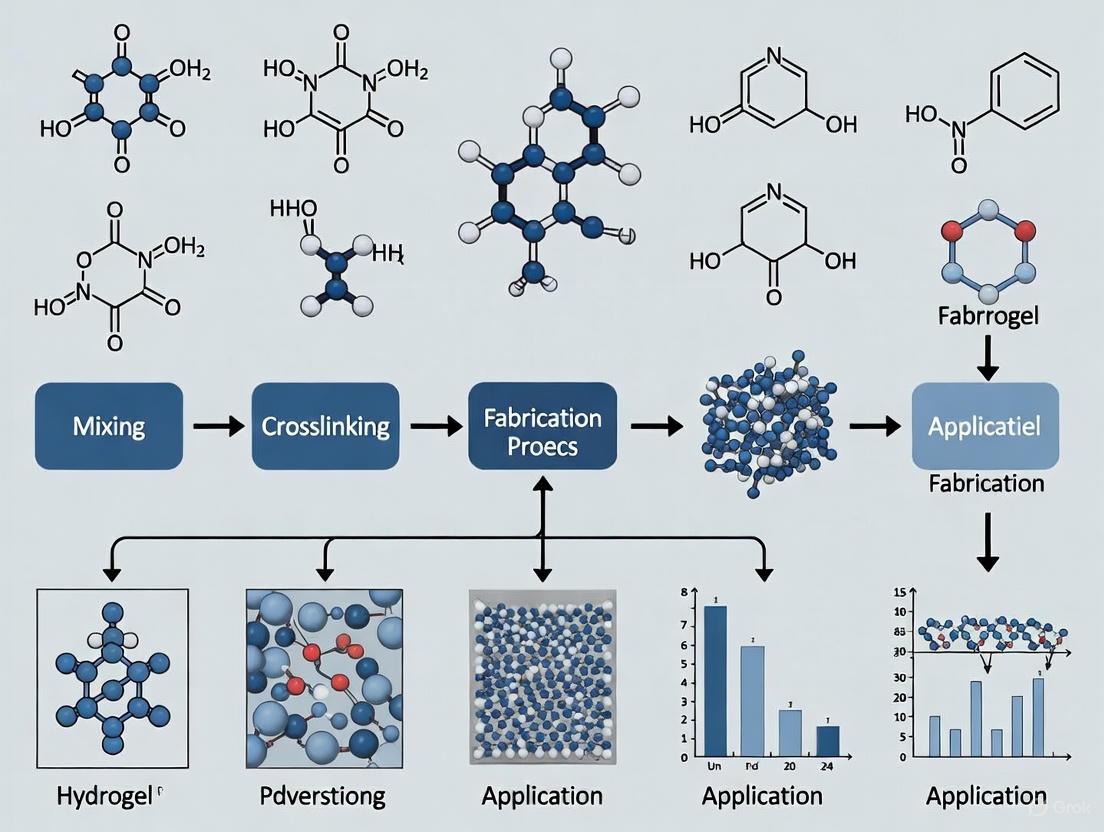
Hydrogels, three-dimensional networks of hydrophilic polymers, have emerged as cornerstone materials in advanced wound care due to their high water content, biocompatibility, and ability to mimic the native extracellular matrix (ECM) [7] [27]. The defining characteristic of any hydrogel—its structural integrity and subsequent functionality in a hydrated state—is dictated by its crosslinking mechanism. Crosslinking describes the process by which polymer chains are interconnected, forming a cohesive network that can swell in water without dissolving. In the context of wound healing, the choice of crosslinking chemistry is not merely a manufacturing consideration; it is a fundamental design parameter that directly influences a hydrogel's mechanical properties, degradation profile, bioactivity, and ultimately, its therapeutic efficacy [28] [29]. This Application Note delineates the primary crosslinking mechanisms employed in hydrogel fabrication for wound healing, provides quantitative comparisons, details standardized experimental protocols, and visualizes critical structure-function relationships to guide research and development.
Classification and Impact of Crosslinking Mechanisms
Hydrogel crosslinking is broadly categorized into physical (reversible) and chemical (permanent) bonds, with advanced hybrid systems combining both approaches to achieve tailored properties [30]. The selection of a crosslinking mechanism profoundly impacts the hydrogel's performance as a wound dressing, influencing critical processes such as cellular infiltration, immunomodulation, and drug release.
Table 1: Comparative Analysis of Primary Hydrogel Crosslinking Mechanisms for Wound Healing
| Crosslinking Type | Bond Nature | Key Characteristics | Impact on Wound Healing Properties | Common Polymers |
|---|---|---|---|---|
| Physical (Reversible) [30] | Non-covalent (H-bonds, ionic, hydrophobic) | Reversible, stimuli-responsive, often injectable, weaker mechanical strength. | Promotes cellular integration and tissue remodeling; allows for minimally invasive application. | Alginate, Chitosan, Gelatin, PVA |
| Chemical (Permanent) [28] [30] | Covalent (C-C, ester, amide) | Permanent, mechanically robust, controlled degradation, risk of cytotoxicity. | Provides structural support for longer durations; enables sustained drug release. | PEG, PVA, GelMA, PAAm |
| Dynamic Covalent [7] | Reversible covalent (e.g., Schiff base, Diels-Alder) | Self-healing, shear-thinning, high mechanical strength. | Extends dressing lifespan; maintains integrity under stress in dynamic wound environment. | Chitosan, Hyaluronic Acid |
The physical properties imparted by crosslinking directly dictate biological outcomes. A seminal study on gelatin methacrylate (GelMA) hydrogels demonstrated that lightly crosslinked (soft) hydrogels promoted greater cellular infiltration and resulted in significantly smaller scars compared to heavily crosslinked (stiff) hydrogels [29]. Heavily crosslinked hydrogels increased inflammation and promoted a pro-fibrotic fibroblast response, underscoring how crosslinking density can guide cellular responses to improve healing.
Experimental Protocol: Fabricating and Evaluating Crosslinked Hydrogels
The following protocol details the synthesis and characterization of a model chemically crosslinked hydrogel system, adaptable for various polymer backbones.
Materials and Reagent Solutions
Table 2: Essential Research Reagents for Hydrogel Crosslinking Studies
| Reagent / Material | Function / Role | Example Specification / Notes |
|---|---|---|
| Gelatin Methacrylate (GelMA) [29] | Photocrosslinkable polymer backbone | Degree of functionalization > 70% |
| Lithium Phenyl-2,4,6-trimethylbenzoylphosphinate (LAP) | Photoinitiator | Enables crosslinking under UV light (365-405 nm) |
| Poly(ethylene glycol) diacrylate (PEGDA) [31] | Synthetic polymer crosslinker | Mn = 700-10,000 Da; defines network mesh size |
| Poly(ethylene glycol) dithiol (PEG-DT) [31] | Crosslinker for Michael addition | Mn = 3,400 Da; reacts with acrylate/vinyl sulfone groups |
| Genipin [30] | Natural, biocompatible chemical crosslinker | Alternative to toxic glutaraldehyde |
| Dulbecco's Phosphate Buffered Saline (DPBS) | Swelling and degradation medium | pH 7.4, isotonic |
Step-by-Step Procedure: Photo-Crosslinking of GelMA Hydrogels
This protocol creates hydrogels with tunable crosslinking density for wound healing applications [29].
- Polymer Solution Preparation: Dissolve GelMA macromer in DPBS at a concentration of 5-15% (w/v) to create a stock solution. Gently heat to 37°C to aid dissolution if necessary.
- Photoinitiator Incorporation: Add the photoinitiator LAP to the GelMA solution at a concentration of 0.1-0.5% (w/v). Protect the solution from light and vortex until fully dissolved.
- Molding and Degassing: Pipette the precursor solution into a polydimethylsiloxane (PDMS) mold or between two glass plates separated by a spacer. Place the mold in a vacuum desiccator for 10-15 minutes to remove air bubbles introduced during mixing.
- UV Light Crosslinking:
- For lightly crosslinked (lo-) GelMA (≈3 kPa): Expose the solution to 365 nm UV light at an intensity of 5-10 mW/cm² for 1 minute.
- For heavily crosslinked (hi-) GelMA (≈150 kPa): Expose the solution to 365 nm UV light at the same intensity for 5 minutes.
- Post-Processing and Sterilization: Carefully extract the crosslinked hydrogel from the mold. For cell culture or in vivo studies, wash the hydrogels three times in sterile DPBS and sterilize under UV light in a biosafety cabinet for 30 minutes per side.
Key Characterization Methods
- Swelling Ratio (Q): Weigh the hydrogel after synthesis (Wd), swell it in DPBS at 37°C for 24-48 hours until equilibrium, then weigh again (Ws). Calculate Q = (Ws - Wd) / Wd. Higher crosslinking density typically results in a lower Q [28].
- Compressive Modulus: Perform uniaxial compression testing on equilibrated hydrogels using a texture analyzer or dynamic mechanical analyzer. The slope of the initial linear region of the stress-strain curve provides the compressive modulus, which correlates directly with crosslinking density [29].
- In Vitro Drug Release: For drug-loaded hydrogels, incubate the hydrogel in a release medium (e.g., DPBS) at 37°C under gentle agitation. Periodically collect release medium and analyze drug concentration via HPLC or UV-Vis spectroscopy. Crosslinking density directly controls release kinetics, from burst to sustained release [31].
Visualization of Crosslinking-Dependent Cell Signaling in Wound Healing
The crosslinking density of a hydrogel dressing directly modulates the behavior of key cells involved in wound repair, such as macrophages and fibroblasts. The following diagram illustrates the distinct signaling pathways activated by soft versus stiff hydrogels.
Figure 1: Cellular Signaling Pathways Modulated by Hydrogel Crosslinking. Soft, lightly crosslinked hydrogels promote a pro-regenerative environment, leading to better healing outcomes. In contrast, stiff, heavily crosslinked hydrogels trigger inflammatory and pro-fibrotic signaling between macrophages and fibroblasts, resulting in increased scarring [29].
The precise engineering of crosslinking mechanisms enables the development of advanced "smart" hydrogels for complex wound management. These include:
- Stimuli-Responsive Drug Release: Hydrogels can be crosslinked to respond to specific wound microenvironment cues (e.g., pH, temperature, or enzyme levels) for on-demand therapeutic agent release [32] [2].
- Self-Healing Hydrogels: Incorporating dynamic reversible bonds (both non-covalent and covalent) allows hydrogels to autonomously repair damage, extending their functional lifespan on dynamic wound beds [7].
- Conductive Hydrogels: Crosslinking conductive polymers (e.g., PEDOT:PSS) or nanomaterials into hydrogel networks facilitates their use as diagnostic dressings for real-time wound monitoring [2].
In conclusion, crosslinking is the foundational process that defines the structure-property-function relationship of hydrogels. A deep understanding of these mechanisms—from simple physical entanglements to complex dynamic covalent networks—is paramount for designing next-generation wound dressings. By strategically selecting the crosslinking chemistry and density, researchers can precisely control hydrogel performance to actively guide the wound healing process toward regeneration, rather than mere repair.
The pursuit of an ideal wound dressing is a central objective in the field of biomedical engineering, particularly within advanced hydrogel fabrication research. The skin, being the largest organ of the human body, serves as a critical physico-chemical barrier against environmental insults, and its impairment necessitates dressings that actively support the complex healing cascade [27] [8]. An optimal dressing must integrate three fundamental properties: superior biocompatibility to interact with biological systems without eliciting adverse responses, effective moisture retention to maintain a hydrated microenvironment conducive to cellular processes, and adequate oxygen permeability to ensure tissue respiration and support various healing phases [33] [34]. Hydrogels, three-dimensional hydrophilic polymer networks, have emerged as a leading class of biomaterials in this domain due to their innate ability to be engineered for these properties, mimicking the native extracellular matrix (ECM) and providing a supportive scaffold for tissue regeneration [27] [35]. This document outlines the core properties of ideal wound dressings, supported by quantitative data, and provides detailed experimental protocols for their evaluation in the context of hydrogel-based wound healing applications.
Core Properties of an Ideal Wound Dressing
The following table summarizes the key properties, their functional significance, and associated quantitative metrics for an ideal wound dressing, with a specific focus on hydrogel-based systems.
Table 1: Key Properties of an Ideal Hydrogel-Based Wound Dressing
| Property | Functional Significance in Wound Healing | Key Quantitative Metrics |
|---|---|---|
| Biocompatibility | Prevents adverse immune reactions, supports cell adhesion, proliferation, and integration with host tissue [27] [8]. | >90% cell viability in ISO 10993-5 cytotoxicity tests [27]; Minimal inflammatory cytokine release (e.g., TNF-α, IL-6) in vitro [33]. |
| Moisture Retention | Maintains a moist wound bed, facilitates autolytic debridement, promotes cell migration, and reduces patient pain [36] [35]. | High Equilibrium Water Content (EWC > 80%) [36]; Water Vapor Transmission Rate (WVTR) of 2000-2500 g/m²/day [34]. |
| Oxygen Permeability | Supports aerobic cellular respiration, neutrophil activity, and angiogenesis while inhibiting anaerobic bacterial growth [33] [34]. | Oxygen diffusion coefficient comparable to native skin (~2.5-5.0 x 10â»â¶ cm²/s) [33]. |
| Mechanical Properties | Provides structural integrity, conforms to wound contours, and withstands mechanical stress during patient movement [27] [30]. | Elastic modulus (E) matching native skin (0.1-20 MPa, depending on location); High elongation at break (>50%) [27]. |
| Bioactivity & Antimicrobial Protection | Actively prevents infection, modulates inflammation, and promotes vascularization and tissue regeneration [36] [30]. | Zone of inhibition >2 mm against common pathogens (e.g., S. aureus, P. aeruginosa); Controlled release of growth factors (e.g., VEGF, FGF) [30]. |
Experimental Protocols for Evaluating Hydrogel Dressing Properties
The following protocols provide standardized methodologies for assessing the critical properties of hydrogel-based wound dressings.
Protocol for Biocompatibility and Cytotoxicity Assessment (ISO 10993-5)
This protocol evaluates the in vitro cytotoxicity of hydrogel extracts using a fibroblast cell line.
Research Reagent Solutions:
- Cell Line: L929 mouse fibroblast cells (ATCC CCL-1)
- Culture Medium: Dulbecco's Modified Eagle Medium (DMEM), supplemented with 10% Fetal Bovine Serum (FBS) and 1% Penicillin-Streptomycin.
- Viability Assay: MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) reagent.
- Extraction Medium: Serum-free DMEM.
- Positive Control: Latex extract.
- Negative Control: High-density Polyethylene (HDPE) extract.
Methodology:
- Hydrogel Extract Preparation: Sterilize the hydrogel sample (e.g., UV irradiation for 30 minutes per side). Using aseptic technique, place a 3 cm² sample per mL of extraction medium in a sterile container. Incubate at 37°C for 24 hours under agitation. Filter the extract through a 0.22 µm filter.
- Cell Seeding: Seed L929 fibroblasts in a 96-well plate at a density of 1 x 10ⴠcells per well in complete culture medium. Incubate for 24 hours at 37°C in a 5% CO₂ atmosphere to form a near-confluent monolayer.
- Sample Exposure: Aspirate the culture medium from the wells. Add 100 µL of the hydrogel extract, positive control, and negative control to respective wells (n=6 per group). Include wells with culture medium only as a blank. Incubate the plate for another 24 hours.
- MTT Assay and Analysis: Add 10 µL of MTT solution (5 mg/mL in PBS) to each well. Incubate for 4 hours. Carefully remove the medium and add 100 µL of dimethyl sulfoxide (DMSO) to solubilize the formed formazan crystals. Measure the absorbance of each well at 570 nm using a microplate reader.
- Data Calculation: Calculate the percentage of cell viability using the formula:
- Cell Viability (%) = (Absorbance of Test Sample / Absorbance of Negative Control) x 100 A cell viability greater than 90% relative to the negative control is considered non-cytotoxic [27].
Protocol for Moisture Retention and Water Vapor Transmission Rate
This protocol determines the hydrogel's water content and its ability to manage moisture at the wound interface.
Research Reagent Solutions:
- Phosphate Buffered Saline (PBS): 0.01 M, pH 7.4.
- Desiccant: Anhydrous calcium chloride.
- Test Setup: Payne cup or similar permeability cup.
Methodology:
- Equilibrium Water Content (EWC):
- Weigh the dry hydrogel sample (Wdry).
- Submerge the sample in PBS at room temperature until swelling equilibrium is reached (no further weight increase).
- Carefully remove the sample, blot gently with filter paper to remove surface water, and weigh immediately (Wwet).
- Calculate EWC using the formula:
- EWC (%) = [(Wwet - Wdry) / W_wet] x 100
- Water Vapor Transmission Rate (WVTR):
- Fill a Payne cup with 10 mL of distilled water.
- Secure the hydrogel sample (of known surface area, A) over the cup opening, ensuring a tight seal.
- Weigh the entire assembly (Winitial) and place it in a controlled environment (e.g., 37°C, 20% relative humidity).
- Weigh the assembly at 24-hour intervals for 3 days (Wfinal).
- Calculate WVTR using the formula:
- WVTR (g/m²/day) = [(Winitial - Wfinal) / (A * T)] where T is the time in days. An ideal range for wound healing is 2000-2500 g/m²/day [34].
Protocol for Oxygen Permeability Measurement
This protocol uses a simplified diffusion cell apparatus to assess the oxygen permeability of hydrogel films.
Research Reagent Solutions:
- Oxygen-Sensitive Probe: Tris(2,2'-bipyridyl)dichlororuthenium(II) hexahydrate.
- Oxygen-Free Nitrogen Gas.
- Sensing Film: Hydrogel film incorporated with the oxygen-sensitive probe.
Methodography:
- Apparatus Setup: Construct a two-chamber diffusion cell where the hydrogel film is mounted as a barrier between a donor chamber (initially filled with nitrogen) and a receiver chamber (filled with PBS saturated with air).
- Data Acquisition: Monitor the partial pressure of oxygen (pOâ‚‚) in the receiver chamber over time using a dissolved oxygen meter or via the fluorescence quenching of the oxygen-sensitive probe.
- Data Analysis: The oxygen permeability coefficient (P) is calculated from the steady-state flux (J) of oxygen across the film, using Fick's first law:
- J = P * (Δp / L) where Δp is the difference in oxygen partial pressure across the film and L is the film thickness. The diffusion coefficient (D) can be derived from the time lag method.
The Scientist's Toolkit: Key Research Reagent Solutions
Table 2: Essential Materials for Hydrogel Wound Dressing Research
| Reagent/Material | Function in Research | Example Applications |
|---|---|---|
| Natural Polymers (e.g., Alginate, Chitosan, Collagen) | Serve as the base scaffold for hydrogels, providing inherent biocompatibility, biodegradability, and bioactivity [27] [8]. | Fabrication of ECM-mimicking scaffolds; creation of hemostatic and antimicrobial dressings [36]. |
| Synthetic Polymers (e.g., PVA, PEG, PLGA) | Provide tunable mechanical strength, controlled degradation rates, and structural stability to the hydrogel network [27] [37]. | Synthesis of high-strength, durable hydrogels; development of stimuli-responsive drug delivery systems [30]. |
| Crosslinkers (e.g., Genipin, EDC/NHS, Glutaraldehyde) | Form stable 3D networks by creating covalent or ionic bonds between polymer chains, determining hydrogel stability and mechanics [30]. | Controlling the swelling ratio, mechanical integrity, and degradation profile of the fabricated hydrogel [27]. |
| Bioactive Agents (e.g., Growth Factors, Antimicrobial Nanoparticles) | Confer advanced therapeutic functions such as promoting angiogenesis or preventing/treating infections [36] [30]. | Engineering drug-eluting dressings for chronic wounds; creating scaffolds with enhanced regenerative capacity [8]. |
| Cell Lines (e.g., L929 Fibroblasts, HaCaT Keratinocytes) | In vitro models for assessing biocompatibility, cytotoxicity, and the ability of the dressing to support cellular functions critical to healing [27] [33]. | Standardized cytotoxicity testing (ISO 10993-5); migration (scratch) assays to simulate re-epithelialization [27]. |
| PC Azido-PEG11-NHS carbonate ester | PC Azido-PEG11-NHS carbonate ester, MF:C42H68N6O21, MW:993.0 g/mol | Chemical Reagent |
| Benzenedimethanamine-diethylamine | Benzenedimethanamine-diethylamine, MF:C16H32N6, MW:308.47 g/mol | Chemical Reagent |
Visualizing the Hydrogel Design Pathway for Ideal Wound Dressings
The following diagram illustrates the logical pathway and key considerations for designing an advanced hydrogel wound dressing that meets the core requirements of biocompatibility, moisture retention, and oxygen permeability.
Hydrogel Design and Evaluation Workflow
This workflow outlines the multi-faceted approach to designing and testing advanced hydrogel dressings, from material selection through to functional application, ensuring all key properties are addressed.
From Lab to Bedside: Fabrication Techniques and Multifunctional Applications
Advanced manufacturing technologies are revolutionizing the design and production of hydrogel-based wound dressings, enabling unprecedented control over material architecture and functionality. These techniques facilitate the creation of personalized, biomimetic constructs that actively support the wound healing process [6]. Traditional wound dressings often act as passive barriers, but advanced manufacturing allows for the development of active systems capable of integrated diagnostics and targeted therapy [6]. This document outlines application notes and experimental protocols for three key advanced manufacturing techniques—3D printing, electrospinning, and micromachining—within the context of hydrogel fabrication for wound healing applications.
Comparative Analysis of Advanced Manufacturing Techniques
The table below summarizes the key characteristics, advantages, and applications of 3D printing, electrospinning, and micromachining in fabricating hydrogels for wound healing.
Table 1: Comparison of Advanced Manufacturing Techniques for Hydrogel-Based Wound Dressings
| Technique | Typical Resolution | Key Advantages | Common Materials | Primary Wound Healing Applications |
|---|---|---|---|---|
| 3D Printing | Micrometer to millimeter scale [38] | High architectural control, patient-specific customization, integration of bioactive components [6] [39] | GelMA, Alginate, PEGDA, HAMA, cellulose derivatives [39] [38] | Custom-shaped dressings for irregular wounds, scaffolds with controlled pore networks for tissue infiltration [6] |
| Electrospinning | Nanometer to micrometer scale (fiber diameter) [40] | High surface area-to-volume ratio, ECM-mimetic nanofibrous structure, efficient drug loading [40] [41] | PCL, Chitosan, Gelatin, Silk fibroin, hybrid polymers [40] | Nanofibrous membranes for exudate management, controlled release of antimicrobials and growth factors [40] [41] |
| Micromachining | Sub-micrometer to micrometer scale [6] | High-precision surface patterning, creation of microfluidic channels and sensor arrays [6] | Various natural and synthetic hydrogels [6] | Integrated biosensors, microneedles for transdermal monitoring, microfluidic systems for biomarker detection [6] |
Experimental Protocols
Protocol 1: Digital Light Processing (DLP) 3D Printing of GelMA-Based Hydrogel Dressings
This protocol describes the fabrication of high-resolution, micropatterned hydrogel patches using DLP 3D printing, suitable for creating personalized wound dressings with enhanced adhesion and antioxidant properties [38].
Research Reagent Solutions
Table 2: Key Reagents for DLP 3D Printing of Hydrogel Dressings
| Reagent | Function | Specifications/Notes |
|---|---|---|
| Gelatin Methacrylate (GelMA) | Primary bioink component providing ECM-mimetic properties and tunable mechanical strength [38] | Synthesized from Type A gelatin (≈300 g Bloom); degree of functionalization should be characterized via 1H NMR [38] |
| Poly(ethylene glycol) diacrylate (PEGDA) | Co-monomer to enhance mechanical properties and printability [38] | Molecular weight (n ≈ 14); helps improve mechanical integrity without significantly compromising cell viability [38] |
| Lithium phenyl(2,4,6-trimethylbenzoyl) phosphinate (LAP) | Photoinitiator for crosslinking under light exposure [38] | Concentration typically 0.5-1.0% (w/v); enables rapid polymerization under 405 nm light [38] |
| Tartrazine (AY 23) | Photoabsorber to control light penetration and enhance printing resolution [38] | Concentration ~0.03% (w/v); prevents over-penetration of UV light, enabling finer feature resolution [38] |
| Gallic Acid (GA) | Functionalization agent for antioxidant activity and improved adhesiveness [38] | Natural polyphenol; post-printing functionalization via EDC/NHS chemistry to scavenge ~80% of free radicals within 4 hours [38] |
Step-by-Step Procedure
Bioink Preparation: a. Prepare a 10% (w/v) solution of GelMA in phosphate-buffered saline (PBS) at 50°C until fully dissolved [38]. b. Add PEGDA co-monomer at a ratio of 1:1 to 1:3 (GelMA:PEGDA) to enhance mechanical properties [38]. c. Incorporate LAP photoinitiator at 0.5% (w/v) and Tartrazine at 0.03% (w/v) into the polymer solution. Mix thoroughly and protect from light [38]. d. Filter the bioink through a 0.22 µm sterile filter if aseptic processing is required.
DLP Printing Process: a. Design the scaffold model using CAD software and convert to an appropriate file format (e.g., STL) [39]. b. Transfer the bioink to the printing reservoir and maintain at 25°C during printing. c. Set printing parameters: layer thickness of 50-100 µm, exposure time of 10-30 seconds per layer depending on light intensity [38]. d. Initiate the printing process. The constructed layers will be photocrosslinked sequentially according to the digital design.
Post-Printing Processing: a. After printing, rinse the constructs with sterile PBS to remove any uncrosslinked material. b. For functionalization with Gallic Acid (GA), prepare a 2 mg/mL GA solution in MES buffer (pH 5.5) with EDC/NHS crosslinkers [38]. c. Immerse the printed constructs in the GA solution for 4-6 hours at room temperature with gentle agitation. d. Wash thoroughly with PBS to remove any unreacted compounds.
Quality Control and Characterization: a. Assess the swelling capacity by measuring weight change after immersion in PBS (typically 200-300% achieved) [39]. b. Evaluate mechanical properties via rheometry to confirm storage modulus (G') values appropriate for wound dressing applications [38]. c. Perform in vitro cytocompatibility testing using fibroblast cell lines (e.g., NHDF) according to ISO 10993-5 standards [38].
Protocol 2: Electrospinning of Nanofibrous Wound Dressing Membranes
This protocol outlines the fabrication of nanofibrous wound dressing membranes using electrospinning technology, creating structures that mimic the native extracellular matrix (ECM) for enhanced wound healing [40] [41].
Research Reagent Solutions
Table 3: Key Reagents for Electrospinning Nanofibrous Dressings
| Reagent | Function | Specifications/Notes |
|---|---|---|
| Polycaprolactone (PCL) | Synthetic polymer backbone providing mechanical strength and controlled biodegradability [40] | Molecular weight ~80,000 Da; provides excellent spinnability and tunable degradation profile [40] |
| Chitosan | Natural polymer imparting antimicrobial activity and biocompatibility [40] | Degree of deacetylation >85%; enhances bioactivity but may require blending with other polymers for improved spinnability [40] |
| Vermiculite Nanoclay | Functional additive to promote angiogenesis and collagen deposition [40] | Two-dimensional nanovermiculite; particularly beneficial for diabetic foot ulcer applications [40] |
| Antimicrobial Agents (e.g., Vanillin) | Bioactive compounds for infection control [39] | Natural antimicrobials like vanillin can be loaded in nanomicelles (2-5% w/w) and incorporated into fibers to avoid bacterial resistance [39] |
Step-by-Step Procedure
Polymer Solution Preparation: a. Prepare a 10-15% (w/v) PCL solution in a 7:3 (v/v) mixture of chloroform and methanol [40]. b. For blended systems, dissolve chitosan in dilute acetic acid (1-2% v/v) and mix with PCL solution at appropriate ratios (typically 3:1 to 1:1 PCL:chitosan) [40]. c. Incorporate functional additives such as vermiculite nanoclay (1-3% w/w) or drug-loaded nanomicelles (2-5% w/w) [40] [39]. d. Stir the solution for 12-24 hours at room temperature to ensure complete homogenization.
Electrospinning Setup: a. Load the polymer solution into a syringe fitted with a metallic needle (gauge 18-22). b. Set the flow rate to 0.5-2.0 mL/hour using a syringe pump. c. Apply high voltage (10-25 kV) between the needle tip and the collector. d. Maintain a working distance of 10-20 cm between the needle and collector. e. Use a rotating mandrel or flat collector based on the desired fiber alignment.
Fiber Collection and Post-processing: a. Collect fibers for 2-6 hours depending on the desired membrane thickness. b. Vacuum-dry the collected nanofibrous membranes at 40°C for 24 hours to remove residual solvents. c. For crosslinking, expose chitosan-containing fibers to glutaraldehyde vapor or UV irradiation as needed.
Characterization and Sterilization: a. Analyze fiber morphology by scanning electron microscopy (SEM); target fiber diameters of 100-500 nm [40]. b. Evaluate porosity, which should be >80% for optimal exudate management and gas exchange [41]. c. Perform antibacterial efficacy testing against common pathogens (e.g., S. aureus and E. coli) following ASTM E2149 standards [39]. d. Sterilize using gamma irradiation or ethylene oxide gas before in vivo applications.
Protocol 3: Micromachining of Hydrogel-Based Sensor Arrays
This protocol describes the use of micromachining techniques to create integrated sensor arrays within hydrogel matrices for real-time monitoring of wound biomarkers, enabling closed-loop wound management systems [6].
Research Reagent Solutions
Table 4: Key Reagents for Micromachined Hydrogel Sensors
| Reagent | Function | Specifications/Notes |
|---|---|---|
| Conductive Additives (MXene, PEDOT:PSS) | Enable real-time sensing of biophysical and biochemical signals [6] | MXene (Ti₃C₂Tₓ) provides high conductivity and biocompatibility; PEDOT:PSS offers stable electrochemical properties [6] |
| Stimuli-Responsive Polymers (PNIPAAm) | Provide temperature-dependent swelling behavior for controlled drug release [6] | Poly(N-isopropyl acrylamide) exhibits reversible volume phase transition at ~32°C; useful for thermo-responsive drug delivery [6] |
| pH-Sensitive Dyes (e.g., Spiropyran) | Enable visual or spectroscopic pH monitoring in wound environment [6] | Spiropyran units enable on-demand antimicrobial activation via photochromism; carboxyl groups provide pH-dependent swelling [6] |
| Enzyme Systems (Glucose Oxidase/Catalase) | Facilitate biochemical sensing and autonomous therapeutic responses [42] | GOx/CAT enzyme pair consumes glucose and regulates local pH; enables feedback-regulated drug release in diabetic wounds [42] |
Step-by-Step Procedure
Hydrogel Formulation for Micropatterning: a. Prepare a base hydrogel precursor solution (e.g., 5-10% GelMA or hybrid polymers) in PBS [6] [38]. b. Incorporate conductive additives (0.5-2% w/w MXene or 3-5% w/w PEDOT:PSS) with thorough mixing and sonication to ensure uniform dispersion [6]. c. Add stimuli-responsive components as required: PNIPAAm (5-10% w/w) for thermoresponsiveness, or spiropyran (0.1-0.5% w/w) for photoresponsive applications [6]. d. For enzymatic feedback systems, incorporate GOx (0.01-0.6 g/L) and catalase (0.08 g/L) into OSA-GEL hydrogels [42].
Micromachining Process: a. Soft Lithography: Create polydimethylsiloxane (PDMS) stamps with desired microchannel patterns (width: 50-200 µm) [6]. b. Pour hydrogel precursor solution onto the patterned substrate or stamp. c. Apply appropriate crosslinking method: UV exposure (for photopolymerizable systems) or ionic crosslinking (for alginate-based systems). d. Laser Ablation: Use focused laser systems for direct writing of microfluidic channels or sensor patterns in pre-formed hydrogel sheets. e. Photolithography: For high-resolution features, use photomasks with UV exposure to define micro-scale patterns in photopolymerizable hydrogels.
Sensor Integration and Calibration: a. Integrate electrodes for electrochemical sensing of pH, glucose, or other biomarkers using screen-printed or micromachined electrode arrays [6]. b. Calibrate pH sensors in buffer solutions across the physiologically relevant range (pH 5.0-8.5) [6] [42]. c. For glucose sensors, calibrate against standard solutions in the range of 1-4 g/L (representing normal to diabetic glucose levels) [42]. d. Validate temperature response for thermoresponsive systems between 25-40°C.
Performance Validation: a. Test sensor response time and sensitivity to target biomarkers in simulated wound fluid. b. Evaluate mechanical compliance of the integrated sensor-hydrogel system to ensure compatibility with skin movement. c. Assess operational stability over 7-14 days in conditions mimicking the wound environment. d. Perform in vitro biocompatibility testing according to ISO 10993-5 standards.
Integrated Manufacturing Strategy for Advanced Wound Care
The convergence of these advanced manufacturing techniques enables the development of next-generation wound dressings with integrated diagnostic and therapeutic functions. A promising approach involves combining 3D-printed structural frameworks with electrospun functional layers and micromachined sensor arrays to create truly intelligent wound management systems [6]. For instance, a 3D-printed alginate-fucoidan scaffold can provide the macroscopic structure and mechanical support [39], while electrospun nanofibers incorporated with antimicrobial nanomicelles offer enhanced infection control [39], and micromachined pH/glucose sensors enable real-time monitoring of wound status [6] [42]. Such integrated systems represent the future of personalized wound care, capable of dynamically adapting treatment strategies based on continuous feedback from the wound microenvironment.
The management of complex wounds, particularly chronic wounds such as diabetic foot ulcers and pressure ulcers, presents a formidable global health challenge, affecting over 40 million patients annually and incurring healthcare costs exceeding $50 billion per year worldwide [1]. Traditional wound dressings, including gauze and hydrocolloids, often fail to address the complex microenvironment of chronic wounds, leading to prolonged healing times and increased risk of complications [1]. In recent years, stimuli-responsive and self-healing hydrogels have emerged as a promising class of biomaterials for advanced wound management due to their unique ability to dynamically adapt to the wound environment and autonomously repair damage [1] [43].
These "smart" hydrogels represent a significant advancement over conventional wound dressings. Their high water content mimics the natural extracellular matrix (ECM), providing a moist environment that facilitates cell proliferation and migration [43] [44]. More importantly, their inherent responsiveness to specific physiological or external stimuli—such as pH, temperature, enzymes, or reactive oxygen species (ROS)—enables precise regulation of therapeutic agent release and functional adaptation [43] [45]. When combined with self-healing capabilities that restore structural integrity after damage, these materials offer unprecedented potential for revolutionizing wound care and regenerative medicine [7] [46].
This application note provides a comprehensive technical resource for researchers and scientists working in hydrogel fabrication for wound healing applications. We summarize key quantitative performance data, detail essential experimental protocols, visualize critical signaling pathways and mechanisms, and catalog fundamental research reagents necessary for advancing the development of next-generation smart wound dressings.
Mechanisms and Performance Characteristics
Stimuli-Responsive Mechanisms in Hydrogels
Stimuli-responsive hydrogels are engineered to undergo reversible or irreversible physical and/or chemical changes in response to specific environmental cues present in wound microenvironments [43]. The table below summarizes the primary stimulus types, their activation triggers in wounds, and the resultant hydrogel responses that facilitate healing.
Table 1: Characteristics and Wound Healing Applications of Stimuli-Responsive Hydrogels
| Stimulus Type | Trigger in Wound Environment | Hydrogel Response | Therapeutic Impact |
|---|---|---|---|
| pH [45] | Alkaline shift (pH ~7.4-9.0) in chronic wounds [45] | Swelling/contraction or degradation; controlled drug release [45] | Targeted antimicrobial delivery; infection control [43] |
| Reactive Oxygen Species (ROS) [43] | Elevated oxidative stress in chronic wounds [43] | Oxidation-triggered disassembly; release of antioxidants or drugs [43] | Scavenging excess ROS; reducing oxidative damage [43] |
| Enzyme [43] | Overexpressed matrix metalloproteinases (MMPs) [43] | Enzyme-sensitive degradation; on-demand drug release [43] | Precise drug delivery at the site of active tissue remodeling [43] |
| Temperature [47] | Skin surface temperature (~32°C) [47] | Sol-gel transition upon contact with body [47] | Facilitates easy application and conformal wound coverage [47] |
| Light [43] | External NIR/UV application [43] | Photothermal or photochemical reactions [43] | Spatiotemporally controlled therapy; biofilm disruption [43] |
Self-Healing Mechanisms and Performance Metrics
Self-healing hydrogels restore their structural integrity and functionality after damage through dynamic, reversible cross-linking mechanisms [7] [46]. These are broadly classified into dynamic covalent bonds and non-covalent interactions, each offering distinct advantages for wound healing applications.
Table 2: Self-Healing Mechanisms and Representative Performance Data
| Healing Mechanism | Chemical Basis | Self-Healing Efficiency | Key Mechanical Properties |
|---|---|---|---|
| Dynamic Covalent Bonds [7] | Schiff base, Diels-Alder, disulfide bonds [7] | >95% recovery within minutes [1] | Moderate strength; excellent for controlled degradation [7] |
| Non-Covalent Interactions [7] | Hydrogen bonding, ionic interactions, host-guest complexes [7] | High efficiency but often lower mechanical strength [7] | Typically soft and elastic; ideal for sensitive wound beds [7] |
| Multimodal Networks [1] | Combination of multiple dynamic bonds [1] | >95% recovery [1] | Enhanced strength (>500% elongation) [1] |
| Nanoconfined Entanglements [48] | Polymer entanglements in co-planar nanosheets [48] | Up to 100% efficiency [48] | Extremely high modulus (50 MPa); tensile strength up to 4.2 MPa [48] |
Experimental Protocols
Protocol: Fabrication of a Schiff Base-Based Self-Healing Hydrogel
Objective: To synthesize an injectable, pH-sensitive self-healing hydrogel using dynamic Schiff base chemistry between modified polymers [7] [1].
Materials:
- Oxidized Alginate (OSA) or other oxidized polysaccharide
- Chitosan (CS) or other amine-rich polymer
- Phosphate Buffered Saline (PBS), pH 7.4
- Deionized Water
Procedure:
- Polymer Preparation:
- Prepare a 2% (w/v) solution of oxidized sodium alginate (OSA) in PBS. Ensure oxidation degree is controlled (typically 20-50%) for optimal bond dynamics.
- Prepare a 2% (w/v) solution of chitosan in dilute acetic acid (1% v/v). Ensure complete dissolution and neutralization to physiological pH if needed.
Hydrogel Formation:
- Mix the OSA and CS solutions in a 1:1 volume ratio under sterile conditions.
- Vortex the mixture gently for 30 seconds. Observe the rapid formation of a hydrogel via the Schiff base reaction between the aldehyde groups on OSA and the amine groups on CS.
- Allow the hydrogel to mature for 30 minutes at room temperature before characterization.
Quality Control:
- Assess gelation time using the vial tilting method.
- Verify self-healing capability by cutting the hydrogel and placing cut surfaces in contact. Visually inspect for reintegration over 1-2 hours.
- Test injectability by extruding the pre-gel solution or freshly formed gel through a standard syringe needle (e.g., 18-22G).
Protocol: Evaluating Self-Healing Efficiency
Objective: To quantitatively and qualitatively assess the self-healing performance of the synthesized hydrogel [46].
Method 1: Macroscopic Visual Assessment
- Prepare two batches of hydrogel, each dyed with a different, biocompatible colorant (e.g., Rhodamine B, Methylene Blue).
- Cut each hydrogel sample into two halves. Recombine halves of different colors to form a single sample with a clear interface.
- Allow the recombined sample to rest undisturbed at room temperature or 37°C for a predetermined healing period (e.g., 1-6 hours).
- Qualitatively evaluate healing by visually inspecting the integrity of the interface. Apply gentle manual stress (e.g., stretching, twisting) to test bond strength. A successfully healed sample should not separate at the interface [46].
Method 2: Rheological Recovery Test
- Instrument Setup: Use a rotational rheometer equipped with a parallel plate geometry (e.g., 20 mm diameter). Load the hydrogel sample and trim excess material.
- Strain Amplitude Sweep: Perform an initial amplitude sweep (e.g., 0.1% - 1000% strain at 1 Hz) to determine the critical strain (γc) where the network structure fractures (identified by the point where G' and G″ cross).
- Alternating Step Strain Test:
- Apply a small oscillatory strain (γ = 1%, well below γc) for 2 minutes to measure the initial storage (G') and loss (G″) moduli.
- Apply a large oscillatory strain (γ = 500%, well above γc) for 1 minute to disrupt the network.
- Immediately switch back to the small strain (γ = 1%) and monitor the recovery of G' and G″ over time.
- Data Analysis: Calculate the healing efficiency (η) using the formula: η = (G'â‚•â‚‘â‚â‚—â‚‘d / G'ᵢₙᵢₜᵢâ‚â‚—) × 100%, where G'â‚•â‚‘â‚â‚—â‚‘d is the storage modulus after recovery and G'ᵢₙᵢₜᵢâ‚â‚— is the initial storage modulus before damage [46].
Signaling Pathways and Experimental Workflows
Hydrogel Stimuli-Responsive Signaling Pathways
The following diagram illustrates the core signaling pathways by which stimuli-responsive hydrogels interact with the wound microenvironment to enable targeted therapeutic action.
Self-Healing Hydrogel Fabrication and Evaluation Workflow
This workflow outlines the key steps involved in the fabrication, characterization, and functional validation of self-healing hydrogels for wound healing applications.
The Scientist's Toolkit: Research Reagent Solutions
Table 3: Essential Materials for Hydrogel Fabrication and Characterization
| Reagent/Material | Function/Application | Examples & Notes |
|---|---|---|
| Natural Polymers [7] [44] | Base material providing biocompatibility and bioactivity. | Chitosan (antibacterial) [7], Hyaluronic Acid (cell migration) [7], Alginate (ion-responsive gelation) [44], Collagen (ECM mimicry) [7]. |
| Synthetic Polymers [44] [47] | Base material offering tunable mechanical properties and modular chemistry. | Polyacrylamide (PAAm) [48], Polyethylene Glycol (PEG) [44], Polyvinyl Alcohol (PVA) [44]. |
| Dynamic Cross-linkers [7] [1] | Enable self-healing and stimuli-responsiveness. | Aldehyde-containing compounds (for Schiff bases) [1], Disulfide-based cross-linkers [7], Phenylboronic acid derivatives (glucose-sensitivity) [43]. |
| Functional Nanomaterials [1] [48] | Enhance mechanical strength, add conductivity, or enable new functions. | Synthetic Hectorite Nanosheets (mechanical reinforcement) [48], MXenes (conductivity) [48], Silver Nanoparticles (antimicrobial) [1], Graphene Oxide (conductivity) [1]. |
| Bioactive Molecules [1] [43] | Provide therapeutic effects to promote wound healing. | Growth Factors (VEGF, EGF) [43], Antimicrobial Peptides (AMPs) [1], Anti-inflammatory drugs (e.g., Gallic acid) [1]. |
| Characterization Tools [46] | Essential for evaluating hydrogel properties and performance. | Rotational Rheometer (self-healing efficiency) [46], Universal Testing Machine (mechanical strength) [48] [46], Scanning Electron Microscope (morphology) [46]. |
| t-Boc-Aminooxy-PEG7-amine | t-Boc-Aminooxy-PEG7-amine, MF:C21H44N2O10, MW:484.6 g/mol | Chemical Reagent |
| Azide-PEG9-amido-C16-Boc | Azide-PEG9-amido-C16-Boc, MF:C42H82N4O12, MW:835.1 g/mol | Chemical Reagent |
Chronic wounds, characterized by a failure to proceed through an orderly and timely healing process, represent a significant clinical and socioeconomic burden globally [33]. The management of these wounds demands innovative strategies that can overcome the pathophysiological barriers to healing, such as persistent inflammation, microbial infection, and impaired tissue regeneration [8]. Hydrogels have emerged as a cornerstone technology in advanced wound care due to their unique capacity to create a hydrated, biocompatible environment that mimics the native extracellular matrix (ECM) [49]. However, the true therapeutic potential of hydrogel platforms is realized through their functionalization with sophisticated bioactive cargos. The integration of growth factors, nanoparticles, and cell secretomes transforms passive hydrogel dressings into active therapeutic systems capable of orchestrating the complex cellular and molecular events necessary for effective wound repair [33] [30]. This document outlines the application and protocol for incorporating these advanced cargos into hydrogel-based wound healing strategies, providing a framework for researchers and drug development professionals.
Hydrogel Platforms for Bioactive Cargo Delivery
Hydrogels provide an ideal microenvironment for wound healing by maintaining moisture, supporting gas exchange, and offering a scaffold for cellular infiltration [33]. Their polymer networks can be engineered from natural sources (e.g., hyaluronic acid, chitosan, collagen) or synthetic materials, each offering distinct advantages in biocompatibility, degradability, and mechanical tunability [8] [49]. The classification of these hydrogels is foundational to selecting the appropriate platform for specific bioactive cargos.
Table 1: Classification and Characteristics of Hydrogels for Wound Healing
| Classification | Crosslinking Mechanism | Key Characteristics | Example Materials | Suitability for Bioactive Cargos |
|---|---|---|---|---|
| Physical Hydrogels [30] | Reversible, non-covalent interactions (e.g., hydrogen bonding, ionic, hydrophobic) | Stimuli-responsive (pH, temperature), injectable, lower mechanical strength | Poloxamer, Alginate-Ca²âº, Polyvinyl alcohol | Suitable for cells, secretomes, and nanoparticles; minimal chemical modification |
| Chemical Hydrogels [30] | Permanent, covalent bonds | Mechanically robust, controlled degradation, long-term stability | Genipin-crosslinked chitosan, PEGDA-based hydrogels | Ideal for sustained release of growth factors and antioxidant nanoparticles |
| Hybrid Hydrogels [30] | Combination of covalent and non-covalent bonds | Balanced mechanical integrity and environmental responsiveness | Ionically and covalently crosslinked alginate | Versatile platform for all cargo types; enables "smart" release kinetics |
Application Notes: Integrating Bioactive Cargos
Nanoparticles
Nanoparticles (NPs) are integrated into hydrogels to confer antimicrobial, anti-inflammatory, and pro-angiogenic properties. Their high surface-area-to-volume ratio enhances the loading capacity and bioavailability of therapeutic agents [30].
Table 2: Nanoparticle-infused Hydrogels for Wound Healing Applications
| Nanoparticle Type | Key Functions & Mechanisms | Hydrogel Composite | Experimental Outcomes |
|---|---|---|---|
| Silver (AgNPs) [8] [30] | Broad-spectrum antimicrobial; reduces microbial burden and biofilm formation | Natural polymer hydrogels (e.g., chitosan, alginate) | Accelerated wound closure, decreased bacterial load in preclinical models |
| Zinc Oxide (ZnONPs) [8] [30] | Antimicrobial, anti-inflammatory, and pro-angiogenic effects | Natural hydrogels | Enhanced granulation tissue formation and re-epithelialization |
| Herbal Self-Assembled NPs (e.g., Naringenin & Curcumin - NC NPs) [50] [51] | Synergistic anti-inflammatory and antioxidant; mitochondrial repair via Nrf2/HO-1 pathway activation and NF-κB suppression | Thermo-sensitive biocompatible hydrogel (NC@Gel) | Suppressed pro-inflammatory factors, promoted macrophage phenotypic transition, accelerated diabetic wound closure in vivo |
| Gold & Cerium Oxide [30] | Potent antioxidant; scavenges reactive oxygen species (ROS) | Synthetic and hybrid hydrogels | Reduced oxidative stress, protected cells from damage, promoted tissue regeneration |
Growth Factors and Cell Secretomes
Growth factors are critical signaling molecules that direct cellular processes such as proliferation, migration, and angiogenesis. The secretome—defined as the complex mixture of bioactive factors (growth factors, cytokines, extracellular vesicles) secreted by cells—offers a multifaceted, cell-free therapeutic approach [52]. Mesenchymal stem cell (MSC) secretomes, in particular, have demonstrated potent immunomodulatory and regenerative effects.
Table 3: Growth Factors and Secretomes in Wound Healing
| Bioactive Cargo | Key Components | Primary Functions in Wound Healing | Hydrogel Delivery System |
|---|---|---|---|
| Growth Factors [8] | VEGF, FGF, TGF-β, PDGF | Angiogenesis, fibroblast proliferation, collagen synthesis, re-epithelialization | Incorporated into hydrogel network for controlled release |
| MSC Secretome [52] | Proteins, cytokines, chemokines, extracellular vesicles (Exosomes, Microvesicles) | Immunomodulation, promotion of cell proliferation & migration, angiogenesis, ECM remodeling | Functionalized biomaterials (e.g., hydrogels, scaffolds) for sustained delivery |
| MSC Source Variants (Adipose, Umbilical Cord, Bone Marrow) [52] | Varying compositions of VEGF, FGF, IGF, HGF | Influences therapeutic efficacy; UC-MSCs and AD-MSCs are preferred for high yield and bioactive content | Pre-conditioning (e.g., hypoxia) can enhance secretome potency before incorporation |
Experimental Protocols
Protocol 1: Fabrication of Herbal Self-Assembled Nanoparticle Hydrogel (NC@Gel)
This protocol describes the preparation of a thermo-sensitive hydrogel loaded with naringenin and curcumin self-assembled nanoparticles (NC NPs) for diabetic wound healing [50] [51].
Research Reagent Solutions
| Reagent/Material | Function | Specifications/Notes |
|---|---|---|
| Naringenin (Nar) | Active pharmaceutical ingredient; anti-inflammatory and antioxidant | Natural flavonoid; ≥95% purity |
| Curcumin (Cur) | Active pharmaceutical ingredient; synergizes with Nar | Natural polyphenol; ≥95% purity |
| Thermo-sensitive polymer (e.g., Poloxamer 407) | Hydrogel matrix | Forms gel at body temperature; biocompatible |
| Solvent (e.g., Ethanol, PBS) | Nanoparticle self-assembly and dispersion | Use sterile, analytical grade |
| Dialysis membrane | Purification of nanoparticles | MWCO 3.5-14 kDa |
Procedure:
- NC NP Self-Assembly:
- Dissolve naringenin and curcumin at a predetermined optimal molar ratio in a suitable solvent (e.g., ethanol) under magnetic stirring at room temperature.
- Slowly add the mixed drug solution dropwise into deionized water under continuous sonication (e.g., 400 W, 10 minutes) to facilitate nanoparticle self-assembly.
- Remove the organic solvent and concentrate the NC NP suspension using a rotary evaporator. Purify the NPs by dialysis against deionized water for 4 hours to remove unloaded drugs. Characterize the NC NPs for size, zeta potential, and drug loading efficiency.
- Hydrogel Loading (NC@Gel Formation):
- Dissolve the thermo-sensitive polymer (e.g., 20% w/v Poloxamer 407) in cold distilled water (4°C) under constant stirring until a clear solution is obtained.
- Gently mix the purified NC NP suspension with the polymer solution on a magnetic stirrer at 4°C, ensuring homogeneous dispersion. Protect from light.
- Sterilize the final NC@Gel formulation by filtration (0.22 µm syringe filter) and store at 4°C until use.
In Vivo Validation:
- Utilize a diabetic mouse (e.g., db/db or STZ-induced) wound model.
- Create full-thickness excisional wounds on the dorsum.
- Apply the NC@Gel topically to the wound bed every other day.
- Monitor wound closure percentage, collect tissue samples for histological analysis (H&E staining for re-epithelialization, Masson's trichrome for collagen), and immunohistochemistry for biomarkers like CD31 (angiogenesis) and NF-κB (inflammation).
Protocol 2: Functionalization of Hydrogels with MSC Secretome
This protocol outlines the process for obtaining the secretome from human MSCs and incorporating it into a hydrogel dressing [52].
Research Reagent Solutions
| Reagent/Material | Function | Specifications/Notes |
|---|---|---|
| Human MSCs | Source of secretome | Adipose tissue (AD-MSC) or umbilical cord (UC-MSC) are preferred |
| Serum-free MSC medium | Cell culture | Avoids contamination with FBS proteins |
| Ultrafiltration units | Secretome concentration | MWCO 3-10 kDa |
| Hydrogel scaffold (e.g., Hyaluronic acid-based) | Delivery vehicle | Can be in sheet or injectable form |
| ELISA Kits | Secretome characterization | Quantify VEGF, FGF, TGF-β |
Procedure:
- Secretome Production:
- Culture human MSCs (from adipose tissue or umbilical cord) to 80% confluence in standard culture flasks.
- Wash cells with PBS and incubate with serum-free medium for 24-48 hours. Pre-conditioning with hypoxia (e.g., 1% Oâ‚‚) can enhance secretome potency.
- Collect the conditioned medium (containing the secretome) and centrifuge (e.g., 2000 × g for 30 min) to remove cell debris.
Secretome Concentration and Preparation:
- Concentrate the supernatant using ultrafiltration units (e.g., 3 kDa MWCO) at 4°C.
- The concentrated secretome can be lyophilized for storage or used directly. Characterize the secretome by ELISA for key growth factors (VEGF, FGF) and nanoparticle tracking analysis for extracellular vesicle concentration.
Hydrogel Functionalization:
- For pre-formed hydrogels: Immerse the sterile hydrogel dressing in the concentrated secretome solution for several hours to allow for passive absorption and diffusion into the matrix.
- For in-situ forming hydrogels: Mix the lyophilized secretome powder or concentrated solution directly into the hydrogel precursor solution before crosslinking.
In Vitro Validation:
- Perform a scratch assay using fibroblasts (e.g., NIH/3T3) to evaluate the effect of the secretome-loaded hydrogel on cell migration.
- Use tube formation assays with human umbilical vein endothelial cells (HUVECs) to assess pro-angiogenic capacity.
The Scientist's Toolkit
Table 4: Essential Research Reagent Solutions for Hydrogel Functionalization
| Category | Item | Specific Function |
|---|---|---|
| Bioactive Cargos | Silver Nanoparticles (AgNPs) | Imparts broad-spectrum antimicrobial properties to prevent infection [30]. |
| Naringenin/Curcumin NPs | Provides synergistic anti-inflammatory and antioxidant effects, targeting mitochondrial dysfunction [50] [51]. | |
| MSC-derived Secretome | Delivers a complex cocktail of growth factors and vesicles for immunomodulation and tissue regeneration [52]. | |
| Hydrogel Polymers | Hyaluronic Acid (HA) | Natural polymer that promotes cell proliferation, angiogenesis, and ECM mimicry [49]. |
| Thermo-sensitive Polymers (e.g., Poloxamer) | Enables formation of injectable hydrogels that gel at body temperature for easy application [30]. | |
| Chitosan | Natural polymer with inherent hemostatic and antimicrobial properties [8]. | |
| Crosslinkers & Agents | Genipin | Biocompatible crosslinker for forming stable, cytocompatible chemical hydrogels [30]. |
| Calcium Chloride (CaClâ‚‚) | Ionic crosslinker for alginate-based physical hydrogels [30]. | |
| Characterization Tools | Nanoparticle Tracking Analyzer | Measures the size and concentration of nanoparticles and extracellular vesicles in secretomes [52]. |
| ELISA Kits | Quantifies specific growth factors and cytokines in secretome formulations [52]. | |
| Rheometer | Characterizes the mechanical and viscoelastic properties of the hydrogel composite [49]. | |
| t-butyl acetate-PEG2-CH2COOH | t-Butyl acetate-PEG2-CH2COOH|PROTAC Linker | t-Butyl acetate-PEG2-CH2COOH is a PEG-based linker for PROTACs synthesis. It connects E3 ligase and target protein ligands. For research use only. Not for human use. |
| N-Boc-N'-(mPEG24)-L-Lysine-OH | N-Boc-N'-(mPEG24)-L-Lysine-OH, MF:C61H120N2O29, MW:1345.6 g/mol | Chemical Reagent |
The management of wound healing, particularly for chronic wounds such as diabetic foot ulcers, remains a significant clinical challenge worldwide. Traditional wound dressings function as passive barriers, offering protection but lacking the capability to actively monitor the healing process or dynamically respond to the wound's microenvironment [53] [54]. This limitation underscores the critical need for advanced therapeutic strategies. The integration of wearable electronics with hydrogel technology has ushered in a new era of intelligent wound care [53] [55]. These smart hydrogels are transforming passive dressings into dynamic, multifunctional platforms capable of real-time physiological monitoring and adaptive therapy, marking a paradigm shift toward personalized and precise wound management [55] [6].
Fundamental Properties of Smart Hydrogels for Sensing
Smart hydrogels are three-dimensional polymer networks that exhibit exceptional moisture retention, biocompatibility, and a structural similarity to the native extracellular matrix, making them ideal for wound contact [53] [54]. Their utility in wearable sensors is driven by a set of engineered properties, which are quantified and summarized in the table below.
Table 1: Key Functional Properties of Sensing Hydrogels and Their Performance Metrics
| Property | Description | Exemplary Materials | Reported Performance |
|---|---|---|---|
| Electrical Conductivity | Enables signal transduction for sensing physiological parameters [53]. | PEDOT:PSS, MXene, Polypyrrole, MWCNTs [56] [55] [6]. | Conductivity: ~0.35 S/m (CS/Gr); Gauge Factor: 0.5–10.97 (HEC/GG) [56]. |
| Mechanical Flexibility & Strength | Allows conformal contact with skin and withstands deformation [57]. | PVA, PAAm, GelMA, HEC/GG/Zinc [53] [56] [55]. | Compressive strength: 97.1 ± 2.8 kPa; Tensile strength up to 54.8 MPa (PEDOT:PSS/PVA) [56] [57]. |
| Self-Healing Ability | Autonomously repairs mechanical damage, enhancing durability [56] [6]. | Dynamic bonds (Schiff base, boronate esters), Zinc ions [56] [6]. | ~78.2% self-healing efficiency without external stimulus [56]. |
| Stimuli-Responsiveness | Dynamically adapts to wound microenvironment changes (pH, temp, enzymes) [53] [55]. | PNIPAAm (thermo-responsive), pH-sensitive carboxyl groups [55] [6]. | Drug release modulated in response to specific wound biomarkers [53]. |
Fabrication Workflow for a Conductive, Self-Healing Hydrogel Sensor
The fabrication of multifunctional hydrogels often involves combining chemical and physical cross-linking strategies to achieve a balance of conductivity, mechanical robustness, and smart functionalities. The following diagram outlines a proven protocol for creating a biomimetic conductive hydrogel.
Diagram 1: Hydrogel Fabrication Workflow. This illustrates the multi-step synthesis of a conductive, self-healing hydrogel via chemical and physical cross-linking [56].
Detailed Experimental Protocol
Title: Fabrication of a Biomimetic Conductive Hydrogel (AHEC/OGG-PDA/Zn²âº) for Wearable Strain Sensing [56].
Objective: To synthesize a multifunctional hydrogel with robust mechanical properties, electrical conductivity, self-healing capability, and 3D printability for application in wearable strain sensors.
Materials:
- Polymers: Hydroxyethyl cellulose (HEC), Guar Gum (GG).
- Cross-linkers & Modifiers: Multi-walled carbon nanotubes (MWCNTs), dopamine, zinc ions (Zn²âº), allyl glycidyl ether.
- Reagents: Cysteamine hydrochloride, 1-ethyl-(3-(3′-dimethylaminopropyl)) carbodiimide (EDC), N-hydroxysuccinimide (NHS).
- Equipment: UV light source (365 nm, 100 W), NMR spectrometer, rheometer, mechanical tester, source meter.
Procedure:
- Polymer Functionalization:
- Allylation of HEC: React HEC with allyl glycidyl ether in a 3:1 mole ratio under alkaline conditions (NaOH, 60°C, 8 hours). Determine the degree of substitution (~0.42) using ( ^1\text{H} ) NMR [56].
- Oxidation and PDA Conjugation of GG: Oxidize GG with sodium periodate to create aldehyde groups. Then, conjugate PDA to the oxidized GG via a Schiff base reaction, facilitated by EDC/NHS chemistry, to form OGG-PDA [56].
- Thiol-Functionalization of MWCNTs: Treat MWCNTs with cysteamine hydrochloride using EDC/NHS coupling to introduce thiol (-SH) groups, creating MWCNTs-SH [56].
Hydrogel Cross-linking:
- Prepare a precursor solution by dissolving the functionalized polymers (AHEC and OGG-PDA) and the conductive cross-linker (MWCNTs-SH) in a suitable solvent.
- Expose the mixture to UV light (365 nm) for 5-10 minutes to initiate the thiol-ene click chemistry reaction, creating a chemically cross-linked network [56].
Ionic Coordination:
- Immerse the cross-linked hydrogel in a solution of zinc sulfate (ZnSO₄) for 24 hours. The Zn²⺠ions diffuse into the network and form coordination bonds with the catechol groups of PDA, introducing a dynamic physical cross-linking component [56].
Characterization and Testing:
- Mechanical Testing: Perform uniaxial compression tests to determine the compressive strength (reported: 97.1 ± 2.8 kPa) [56].
- Self-Healing Test: Cut the hydrogel completely and bring the cut surfaces into contact. Monitor the recovery of mechanical integrity and electrical conductivity over time without external stimulus. Calculate self-healing efficiency (reported: 78.2%) [56].
- Sensing Performance: Connect the hydrogel to a source meter and measure resistance changes while applying cyclic strains. Calculate the gauge factor (GF = (ΔR/R₀)/ε, where R is resistance and ε is strain), which ranges from 0.5 to 10.97 for this formulation [56].
The Scientist's Toolkit: Essential Research Reagents and Materials
The development and fabrication of advanced hydrogel sensors rely on a specific set of materials. The following table catalogs key reagents and their functions for researchers.
Table 2: Essential Research Reagents for Hydrogel Sensor Fabrication
| Reagent/Material | Function/Application | Key Characteristics |
|---|---|---|
| Natural Polymers (Chitosan, Hyaluronic Acid, Alginate) [55] [58] | Base matrix providing biocompatibility and biodegradability. | Inherent bioactivity, mimics the extracellular matrix [55]. |
| Synthetic Polymers (PVA, PAAm, PEG) [53] [55] | Enhances mechanical strength and stability of the hydrogel network. | Tunable mechanical properties and chemical stability [55]. |
| Conductive Fillers (MWCNTs, PEDOT:PSS, MXene) [56] [55] | Creates conductive pathways for sensing and electrical stimulation. | High electrical conductivity, can be functionalized for better dispersion [56]. |
| Dynamic Cross-linkers (Zinc Ions, Schiff base formers) [56] [6] | Enables self-healing and injectability through reversible bonds. | Forms ionic or dynamic covalent bonds that can break and reform [56]. |
| Stimuli-Responsive Moieties (PNIPAAm, pH-sensitive groups) [55] | Confers smart, on-demand drug release in response to wound biomarkers. | Undergoes conformational or property changes with pH, temperature, etc. [55]. |
| Bisabola-2,10-diene-1,9-dione | Bisabola-2,10-diene-1,9-dione, CAS:83217-93-0, MF:C15H22O2, MW:234.33 g/mol | Chemical Reagent |
| 5-HydroxyMebendazole-D3 | 5-Hydroxymebendazole D3|CAS 1173020-86-4 | 5-Hydroxymebendazole D3 (CAS 1173020-86-4) is a deuterated metabolite standard for precise analytical research. This product is for Research Use Only and is not intended for diagnostic or therapeutic use. |
Application Notes: Intelligent Closed-Loop Wound Management
The ultimate application of wearable hydrogel sensors is in integrated, closed-loop systems that diagnose and treat wounds autonomously. The a-Heal platform exemplifies this concept, merging wearable devices with an AI-driven controller for adaptive therapy [59].
Diagram 2: Closed-Loop Wound Management. This illustrates the autonomous diagnostic and therapeutic cycle of an intelligent wound care platform [59].
Protocol for Implementing a Closed-Loop Diagnostic and Therapeutic Cycle
System Overview: The a-Heal platform is a portable, wireless system for real-time, adaptive bioelectronic wound therapy. It integrates a wearable device for wound imaging and therapy delivery with an ML Physician for automated decision-making [59].
Components:
- Wearable Device: Contains a camera module, illumination ring, transparent PDMS body housing drug reservoirs and bioelectronic actuators (e.g., for iontophoretic drug delivery or electric field application), and a microcontroller for wireless communication [59].
- ML Physician Algorithm: Comprises a Deep Mapper (an Autoencoder coupled with a linear dynamics model) to map wound images to a state space representing healing stages, and a Deep Reinforcement Learning (DRL) controller to determine optimal treatment parameters [59].
Operational Cycle:
- Image Acquisition and Diagnosis:
- The wearable device's camera captures a z-stack of wound images, which are transmitted wirelessly to the ML Physician [59].
- The ML Physician's Deep Mapper encodes the image into a four-state vector (z_k), representing the probability of the wound being in each of the four key healing stages: hemostasis, inflammation, proliferation, and maturation [59].
- This state is compared to an optimal healing trajectory.
Therapy Prescription and Delivery:
- The DRL controller (the "follower") uses a reward function based on the distance between the actual wound state and the optimal state to calculate the best therapeutic action [59].
- The prescribed therapy (e.g., specific electric field intensity or fluoxetine dosage) is sent wirelessly back to the wearable device [59].
- The device's bioelectronic actuators deliver the therapy, for example, by applying a voltage for iontophoretic drug release or electric field stimulation [59].
Cycle Repetition:
Chronic wounds, including diabetic ulcers, burns, and pressure injuries, represent a significant clinical challenge with substantial socioeconomic burdens on global healthcare systems [60] [4]. These wounds are characterized by prolonged inflammation, increased risk of infection, and dysregulated tissue repair processes that prevent normal healing progression [61] [33]. Hydrogel-based wound dressings have emerged as versatile biomaterials that actively support the healing process through their unique physicochemical properties, including high water content, biocompatibility, tunable mechanical characteristics, and functionality as drug delivery platforms [60] [6] [33]. This Application Note provides a comprehensive framework for researchers developing hydrogel-based therapies for complex wound management, with specific protocols for fabrication, functionalization, and efficacy assessment targeting the distinct pathophysiological features of diabetic ulcers, burns, and pressure injuries.
Hydrogel Efficacy Data for Complex Wounds
Table 1: Clinical Efficacy of Hydrogel Dressings Across Wound Types
| Wound Type | Study Design | Healing Outcomes | Comparative Effectiveness | References |
|---|---|---|---|---|
| Diabetic Foot Ulcers | Systematic review of clinical trials | Increased rate of complete wound healing; Enhanced wound size reduction | Ranked 3rd most suitable after hydrocolloid matrices and foam dressings | [4] |
| Burn Wounds | Clinical trials | Reduced healing time; Enhanced re-epithelialization | Superior to silver sulphadiazine in partial thickness burns | [4] |
| Pressure Ulcers | Network meta-analysis | Improved wound closure; Reduced healing time | Variable efficacy compared to other advanced dressings | [4] |
| Infected Wounds | Animal study (mouse model) | Accelerated closure of wounds infected with multi-drug resistant P. aeruginosa; 65% reduction in mature biofilm | Significant antibacterial action and improved wound healing | [62] |
Table 2: Functional Capabilities of Advanced Hydrogel Systems
| Hydrogel Function | Active Components | Mechanism of Action | Target Wound Type | References |
|---|---|---|---|---|
| Glycemic Control | Glucose-responsive materials | On-demand insulin release in response to elevated glucose | Diabetic wounds | [60] |
| Nerve Regeneration | A-lipoic acid, growth factors | Enhanced peripheral nerve perfusion; Alleviation of oxidative damage | Diabetic peripheral neuropathy | [60] |
| Vascular Regeneration | VEGF, platelet-derived growth factor | Stimulation of angiogenesis; Improved microcirculation | Diabetic wounds, Pressure injuries | [60] |
| Anti-infective | Silver nanoparticles, nitric oxide, ciprofloxacin | Disruption of bacterial cell membranes; Biofilm prevention; Controlled antibiotic release | Infected burns, Chronic wounds | [62] |
| Bone Repair | Osteoinductive factors | Promotion of bone regeneration in deep wounds | Complex wounds with bone exposure | [60] |
Experimental Protocols
Protocol 1: Fabrication of Multifunctional Hydrogel with Antimicrobial Properties
This protocol describes the synthesis of a chitosan-based hydrogel loaded with silver nanoparticles (AgNPs) and ciprofloxacin for managing infected wounds, particularly effective against multi-drug resistant Pseudomonas aeruginosa [62].
Materials:
- Chitosan (medium molecular weight)
- Acetic acid (3% v/v aqueous solution)
- S-nitrosoglutathione (GSNO)-poly(vinyl methyl ether co-maleic anhydride) (PVMMA) solution (5% w/v)
- Silver nanoparticles (AgNPs, 20-50 nm)
- Ciprofloxacin hydrochloride
- Deionized water
- Pyrex petri dishes
Method:
- Prepare a 2% (w/v) chitosan solution by dissolving chitosan in 3% (v/v) acetic acid with continuous stirring for 2 hours at room temperature.
- Prepare the GSNO-PVMMA solution (5% w/v) by dissolving GSNO-PVMMA in distilled water with continuous stirring at 50°C for 4 hours.
- Add AgNPs (0.1% w/w relative to final hydrogel weight) and ciprofloxacin (1-2% w/w) to the GSNO-PVMMA solution with vigorous stirring.
- Combine chitosan and GSNO-PVMMA solutions in a 4:1 ratio using mechanical blending at 1000 rpm for 10 minutes.
- Pour the mixture into Pyrex petri dishes and air-dry at room temperature for 48 hours.
- Remove dried hydrogel scaffolds from Petri dishes and store in a desiccator until use.
Characterization:
- Swelling Studies: Determine equilibrium swelling ratio (ESR) in PBS at different temperatures using gravimetric method: ESR = Ww/Wd, where Ww and Wd are weights of swollen and dried hydrogel, respectively.
- Microstructure Analysis: Examine ultrastructure using scanning electron microscopy (SEM) at 20 kV accelerating voltage after gold coating.
- FT-IR Analysis: Confirm chemical composition using Fourier-transform infrared spectroscopy in the range of 4000-400 cmâ»Â¹.
- Thermal Analysis: Perform thermogravimetric analysis (TGA) and differential thermal analysis (DTA) from room temperature to 900°C at a heating rate of 10°C/min under nitrogen atmosphere.
Protocol 2: In Vitro Assessment of Anti-biofilm Activity
Materials:
- Test organism (e.g., multi-drug resistant P. aeruginosa)
- Mueller-Hinton broth
- 96-well flat-bottom polystyrene microtiter plates
- Crystal violet solution (0.1% w/v)
- Acetic acid (30% v/v)
- Microplate reader
Method:
- Prepare bacterial suspension equivalent to 0.5 McFarland standard in Mueller-Hinton broth.
- Add 100 μL of bacterial suspension to wells of microtiter plate.
- Add hydrogel extracts at concentrations ranging from 1× to 0.25× MIC.
- Incubate plates at 37°C for 24 hours.
- Carefully remove planktonic cells and wash wells with phosphate buffered saline (PBS).
- Fix adherent cells with 99% methanol for 15 minutes, then stain with 0.1% crystal violet for 5 minutes.
- Wash off excess stain and solubilize bound dye with 30% acetic acid.
- Measure optical density at 595 nm using microplate reader.
- Calculate percentage biofilm inhibition relative to untreated control.
Protocol 3: In Vivo Evaluation of Wound Healing Efficacy
Materials:
- Animal model (e.g., 25 rats for burn wound infection study)
- Ketamine/xylazine anesthetic cocktail
- Burn wound creation apparatus
- Bacterial suspension of MDR P. aeruginosa (10⸠CFU/mL)
- Test hydrogel dressings
- Histopathology supplies (formalin, paraffin, H&E stain)
Method:
- Anesthetize animals using appropriate anesthetic protocol.
- Create standardized burn wounds on dorsal surface.
- Infect wounds with 10⸠CFU/mL of MDR P. aeruginosa.
- Apply test hydrogel dressings daily to wound area.
- Monitor wound closure percentage through digital photography and image analysis at regular intervals.
- Sacrifice animals at predetermined endpoints for histopathological analysis.
- Process tissue samples for H&E staining to assess epithelial thickness, granulation tissue formation, and inflammatory cell infiltration.
- Compare healing parameters between treatment groups using appropriate statistical methods.
Advanced Applications and Engineering Strategies
Nanoparticle-Infused Hydrogel Systems
The integration of nanoparticles into hydrogel matrices enhances their therapeutic potential through improved antimicrobial activity, controlled drug release, and tissue regeneration capabilities [30] [61]. Different nanoparticle classes offer distinct advantages:
Table 3: Nanoparticle Classes for Hydrogel Functionalization
| Nanoparticle Class | Representative Materials | Key Functions | Wound Applications |
|---|---|---|---|
| Metallic | Silver, Zinc Oxide, Gold | Antimicrobial, Anti-inflammatory, Antioxidant | Infected wounds, Diabetic ulcers |
| Carbon-based | Graphene oxide, Carbon nanotubes, Carbon dots | Antibacterial, Conductivity, Tissue regeneration | Chronic wounds, Monitoring applications |
| Polymeric | Chitosan, Poly(lactic-co-glycolic acid), Polycaprolactone | Controlled drug release, Accelerated wound closure | All wound types, Drug delivery |
| Cellulosic | Cellulose nanofibrils, Cellulose nanocrystals | Improved mechanical properties, Cell proliferation, Hemostasis | Pressure injuries, Exuding wounds |
| Lipid-based | Solid lipid nanoparticles, Nanostructured lipid carriers, Liposomes | High entrapment efficiency, Controlled delivery, Antibacterial enhancement | Dry wounds, Drug delivery |
Intelligent Hydrogel Systems with Closed-Loop Feedback
Advanced hydrogel platforms integrate diagnostic and therapeutic functions through responsive material systems that adapt to wound microenvironment changes [6]. These "smart" hydrogels can be engineered to respond to specific wound biomarkers:
Hydrogel Crosslinking Strategies and Their Applications
The crosslinking mechanism fundamentally determines hydrogel properties and applications. Researchers can select from three primary approaches:
The Scientist's Toolkit: Research Reagent Solutions
Table 4: Essential Research Materials for Hydrogel Wound Dressing Development
| Reagent Category | Specific Examples | Function in Hydrogel System | Application Notes |
|---|---|---|---|
| Natural Polymers | Chitosan, Alginate, Hyaluronic acid, Gelatin, Collagen, Cellulose derivatives | Structural backbone, Biocompatibility, Bioactivity | Chitosan provides inherent antimicrobial properties; Alginate offers high absorbency |
| Synthetic Polymers | Poly(ethylene glycol), Poly(vinyl alcohol), Polyacrylamide, Poloxamers | Mechanical stability, Controlled degradation, Tunable properties | PEG enables controlled drug release; PVA enhances mechanical strength |
| Crosslinkers | Genipin, Glutaraldehyde, EDC/NHS, Diglycidyl ethers | Network formation, Structural integrity | Genipin offers lower cytotoxicity than glutaraldehyde; EDC/NHS for carbodiimide chemistry |
| Therapeutic Agents | Silver nanoparticles, Ciprofloxacin, Nitric oxide donors, VEGF, Growth factors | Antimicrobial action, Tissue regeneration, Angiogenesis | AgNPs provide broad-spectrum antimicrobial activity; VEGF promotes vascularization |
| Functional Additives | Graphene oxide, Carbon nanotubes, Cellulose nanocrystals, Conductive polymers | Enhanced mechanical properties, Conductivity, Sensing capabilities | GO improves mechanical strength and offers antibacterial properties |
| Manufacturing Aids | Photoinitiators (Irgacure 2959), Thermal initiators (APS/TEMED) | Polymerization initiation, Gelation control | Irgacure 2959 for UV crosslinking; APS/TEMED for thermal initiation systems |
| Heptadecan-9-yl 8-bromooctanoate | Heptadecan-9-yl 8-bromooctanoate, CAS:2089253-22-3, MF:C25H49BrO2, MW:461.6 g/mol | Chemical Reagent | Bench Chemicals |
| Droxicainide hydrochloride | Droxicainide hydrochloride, CAS:78289-16-4, MF:C16H25ClN2O2, MW:312.83 g/mol | Chemical Reagent | Bench Chemicals |
Hydrogel-based therapies represent a promising frontier in the management of complex wounds including diabetic ulcers, burns, and pressure injuries. The protocols and data presented in this Application Note provide researchers with validated methodologies for developing and evaluating advanced hydrogel systems with targeted therapeutic functionalities. Future directions in the field include the development of fully integrated closed-loop systems that combine real-time monitoring with automated therapeutic responses, personalized hydrogel platforms tailored to individual patient wound microenvironments, and the incorporation of artificial intelligence for predictive wound management. As research advances, hydrogel technologies are poised to transform wound care from passive coverage to active, intelligent management systems that significantly improve clinical outcomes for patients with complex wounds.
Navigating Challenges: Strategies for Optimizing Hydrogel Performance and Safety
The development of advanced hydrogels for wound healing represents a significant frontier in biomedical science, particularly for managing complex chronic wounds such as diabetic foot ulcers and venous leg ulcers. These non-healing wounds affect over 40 million patients globally annually, creating healthcare costs exceeding $50 billion worldwide [1]. Hydrogels, three-dimensional networks of hydrophilic polymers with high water content, have emerged as promising wound dressing materials due to their biocompatibility, ability to maintain a moist wound environment, and capacity for targeted therapeutic delivery [8] [33]. However, a fundamental challenge persists: how to enhance the often-limited mechanical strength of hydrogels without diminishing their essential bioactivity—the very property that enables positive interactions with biological systems and promotes healing [63].
This challenge stems from the inherent structural trade-offs in hydrogel design. Natural hydrogels, derived from sources like collagen, chitosan, hyaluronic acid, and alginate, possess inherent bioactivity, enzymatic degradability, and cell-adhesive qualities that closely mimic the native extracellular matrix (ECM) [8] [64]. These features facilitate crucial cell interactions, modulate inflammation, and accelerate tissue remodeling but often provide insufficient mechanical strength and stability for clinical handling and sustained function in dynamic wound environments [63]. Conversely, synthetic hydrogels, typically fabricated from polymers like polyethylene glycol (PEG), polyvinyl alcohol (PVA), and poly-N-isopropylacrylamide (PNIPAAm), offer superior mechanical tunability and stability but lack inherent bioactivity and cellular recognition sites [64] [63].
This Application Note details strategic design approaches and experimental protocols to navigate this critical balance, enabling the creation of hydrogel platforms that are both mechanically robust and biologically active for advanced wound healing applications.
Quantitative Landscape: Material Properties and Performance
Table 1: Comparison of Natural and Synthetic Hydrogel Components for Wound Healing
| Polymer Type | Example Materials | Tensile Strength/Elastic Modulus | Key Bioactive Properties | Primary Limitations |
|---|---|---|---|---|
| Natural Hydrogels | Collagen, Chitosan, Hyaluronic Acid, Fibrin, Gelatin | Low elastic moduli (e.g., <1 kPa for some structures) [65]; Varies with crosslinking | Inherent biocompatibility; cell adhesion motifs; enzymatic degradation; immunomodulation [8] [64] | Low mechanical strength; batch-to-batch variability; rapid degradation [63] |
| Synthetic Hydrogels | PEG, PVA, PVP, PNIPAAm, PAM | Wide range achievable; Enhanced by crosslinking (e.g., PVA) [63] | Highly tunable chemistry; predictable mechanical properties; long-term stability [64] [63] | Lack of cell-adhesive sites; potential cytotoxicity of degradation products; bioinert [63] |
Table 2: Advanced Hydrogel Systems and Their Resolved Properties
| Hydrogel System | Design Strategy | Mechanical Outcome | Bioactive Outcome |
|---|---|---|---|
| Self-Healing Hydrogels [1] | Dynamic bonds (e.g., Schiff base, hydrogen bonds, metal coordination) | Autonomous repair of damage; restoration of mechanical integrity after deformation | Maintains physical barrier; extends dressing lifespan; reduces need for replacement |
| Gradient Hydrogels [66] | Spatial variation in crosslink density, composition, or porosity | Graded mechanical properties mimicking tissue interfaces; enhanced toughness | Directional cell migration and tissue integration; spatially controlled drug release |
| Hierarchically Ordered Porous Hydrogels [65] | Bottom-up self-assembly of triblock copolymers | High elasticity (extending >12x initial length); hyperelasticity; completely reversible deformation [65] | Porous microstructure (≈98% water) conducive to cell proliferation and nutrient transport [65] |
| Multifunctional Bilayer Dressings [34] | Hydrogel layer combined with electrospun nanofiber layer | Dense nanofiber layer provides mechanical protection and integrity | Hydrogel layer maintains moist environment and supports cell growth |
Table 3: Key Characterization Techniques for Mechanical and Bioactive Properties
| Property Category | Characterization Technique | Key Measurable Parameters |
|---|---|---|
| Mechanical Properties | Rheometry, Dynamic Mechanical Analysis (DMA) [63] | Elastic modulus (G'), Viscous modulus (G''), Complex modulus, Tensile strength, Elongation at break |
| Structural & Morphological Properties | Scanning Electron Microscopy (SEM), Micro-CT, Small-Angle X-Ray Scattering (SAXS) [63] | Pore size, porosity, interconnectivity, fibrillar structure, network homogeneity |
| Chemical & Swelling Properties | FTIR Spectroscopy, NMR, Swelling Ratio Studies [62] [63] | Functional groups, crosslinking density, equilibrium swelling ratio (ESR), degradation kinetics |
Strategic Frameworks and Experimental Pathways
Core Design Principles for Balanced Hydrogels
Achieving the balance between mechanical strength and bioactivity requires moving beyond simple homogeneous networks. The following design principles, derived from recent advances, provide a framework for success:
Principle of Multifunctional Dynamic Networks: Incorporate multiple, reversible crosslinking mechanisms (e.g., hydrogen bonds combined with Schiff base linkages or metal coordination) to create hydrogels that are both strong and self-healing. This approach distributes stress throughout the network and allows for autonomous repair of damage, maintaining structural integrity under the dynamic conditions of a wound bed [1]. Studies have demonstrated that dual-dynamic networks can achieve mechanical strengths exceeding 500% elongation with self-healing efficiencies over 95% recovery within minutes [1].
Principle of Structural Hierarchy: Engineer hydrogels with ordered structures across multiple length scales (nano to micro) to mimic natural tissues. A prime example is the creation of hierarchically ordered hydrogel fibers featuring nanostructured micelle walls forming micrometer-sized, water-rich pores. This structure results in unique mechanical properties, including low elastic moduli (<1 kPa), high elasticity (extending by more than 12 times their initial length), hyperelasticity, and completely reversible deformation—all while maintaining a hydrous environment conducive to cell proliferation [65].
Principle of Bioactive Reinforcement: Enhance mechanical properties by incorporating bioactive fillers rather than inert ones. For instance, the addition of antimicrobial nanoparticles like silver (AgNPs) or regenerative agents like growth factors not only strengthens the hydrogel network but also introduces targeted therapeutic functions [8] [62]. This creates a synergistic effect where the composite material's mechanical and biological properties are simultaneously enhanced.
Visualizing the Integrated Design Strategy
The following diagram illustrates the multi-faceted approach required to balance mechanical and bioactive properties in hydrogel design, integrating material selection, structural engineering, and functional enhancement.
Application Notes & Experimental Protocols
Protocol 1: Fabrication of a Self-Healing, Multifunctional Hydrogel
This protocol details the synthesis of an injectable, self-healing hydrogel combining chitosan and functionalized hyaluronic acid, designed for treating chronic wounds. The dynamic Schiff base bonds formed between amine and aldehyde groups provide both self-healing properties and a biocompatible, cell-supportive environment [1].
Research Reagent Solutions:
Table 4: Essential Reagents for Self-Healing Hydrogel Fabrication
| Reagent/Material | Function/Explanation | Exemplar Source/Type |
|---|---|---|
| Chitosan | Natural polymer providing primary backbone and free amine groups for cross-linking and inherent antimicrobial activity. | Commercial source, degree of deacetylation >85% |
| Sodium Hyaluronate (HA) | Natural ECM component providing hydrophilicity, cell interaction sites, and backbone for chemical modification. | Commercial source, pharmaceutical grade |
| Periodate (NaIOâ‚„) | Oxidizing agent for cleaving the vicinal diols of HA to create aldehyde groups (-CHO) for Schiff base formation. | Sigma-Aldrich, ACS reagent grade |
| Phosphate Buffered Saline (PBS) | Physiological buffer for dissolving and adjusting the pH of polymer solutions. | Thermo Fisher Scientific, pH 7.4 |
| Glycerol | Plasticizer added to modulate the final mechanical properties and prevent brittleness. | MilliporeSigma, ≥99% |
Step-by-Step Procedure:
Synthesis of Oxidized Hyaluronic Acid (OHA):
- Dissolve 1.0 g of sodium hyaluronate in 100 mL of deionized water under gentle stirring at room temperature, protected from light.
- Dissolve 0.8 g of sodium periodate (NaIOâ‚„) in 10 mL of deionized water and add it dropwise to the HA solution.
- Allow the reaction to proceed for 4 hours at room temperature with continuous stirring in the dark.
- Terminate the reaction by adding 1 mL of ethylene glycol and stir for an additional 30 minutes.
- Purify the OHA product by dialysis (MWCO 3.5 kDa) against deionized water for 48 hours, with frequent water changes.
- Recover the final product by freeze-drying and store at -20°C. Characterize the degree of oxidation via 1H-NMR.
Preparation of Chitosan Solution:
- Dissolve 2.0 g of chitosan in 100 mL of a 1% (v/v) acetic acid solution. Stir vigorously until a clear, homogeneous solution is obtained.
- Adjust the pH to approximately 6.5 using 1M NaOH solution to ensure the amine groups are available for reaction without causing gelation.
Hydrogel Formation and In-Situ Gelation:
- Prepare separate aqueous solutions of OHA (4% w/v) and Chitosan (2.5% w/v) in PBS.
- For gelation, mix the OHA and Chitosan solutions in a 1:1 volume ratio. The gel point is typically reached within seconds to minutes at room temperature, facilitated by the formation of Schiff base linkages between the aldehyde groups of OHA and the amine groups of chitosan.
- The resulting hydrogel can be loaded into a syringe for injectable application to an irregular wound bed.
Validation and Characterization:
- Self-Healing Test: Visually observe and record the fusion of two dyed hydrogel pieces placed in contact. Quantify recovery of mechanical properties by rheology, measuring the storage modulus (G') recovery after successive strain sweeps (e.g., 1% strain for recovery, 500% strain for rupture) [1].
- Rheological Analysis: Perform oscillatory time sweeps and amplitude sweeps to determine the storage modulus (G'), loss modulus (G''), and critical strain.
- Swelling Study: Measure the equilibrium swelling ratio (ESR) in PBS at 37°C using the formula: ESR = (Ww - Wd) / Wd, where Ww and W_d are the weights of the swollen and dry hydrogel, respectively [62].
- Antimicrobial Assay: Evaluate efficacy against common pathogens like Pseudomonas aeruginosa using a zone of inhibition test or by assessing reduction in bacterial viability in co-culture [62].
Protocol 2: Fabrication of a Mechanically Reinforced, Bioactive Nanocomposite Hydrogel
This protocol creates a chitosan-based hydrogel matrix reinforced with silver nanoparticles (AgNPs) and loaded with ciprofloxacin, providing robust mechanical properties, antimicrobial activity, and controlled drug release for infected wound management [62].
Research Reagent Solutions:
Table 5: Essential Reagents for Nanocomposite Hydrogel Fabrication
| Reagent/Material | Function/Explanation | Exemplar Source/Type |
|---|---|---|
| Chitosan | Primary biopolymer matrix; provides cationic sites, biocompatibility, and inherent hemostatic property. | Commercial source, medium molecular weight |
| Silver Nanoparticles (AgNPs) | Bioactive filler providing mechanical reinforcement and broad-spectrum antimicrobial activity. | Pre-synthesized, ~20-40 nm diameter, stabilized |
| Ciprofloxacin HCl | Broad-spectrum antibiotic model drug for localized treatment of wound infection. | Sigma-Aldrich, USP grade |
| Acetic Acid | Solvent for dissolving chitosan. | Fisher Chemical, glacial, ACS grade |
| Glycerol | Plasticizer to improve flexibility and handling properties of the final hydrogel film. | MilliporeSigma, ≥99% |
| Poly(Vinyl Methyl Ether Co-Maleic Anhydride) (PVMMA) | Used here as a model crosslinker/compatibilizer to enhance stability and integrate components. | Sigma-Aldrich |
Step-by-Step Procedure:
Polymer Solution Preparation:
- Dissolve 2.0 g of chitosan in 100 mL of a 3% (v/v) acetic acid solution with continuous stirring for 2 hours until fully dissolved.
- In a separate vessel, prepare a 5% (w/v) solution of PVMMA in distilled water by stirring at 50°C for 4 hours.
Nanocomposite Integration and Gel Formation:
- To the PVMMA solution, add AgNPs (target final concentration 0.1-1 mg/mL in hydrogel) and ciprofloxacin (target final concentration 1-5 mg/mL in hydrogel). Stir the mixture thoroughly to ensure homogeneous dispersion.
- Combine the chitosan solution and the PVMMA/AgNP/Ciprofloxacin mixture in a 4:1 volume ratio. Blend mechanically at 1000 rpm for 10 minutes.
- Pour the resulting solution into Petri dishes and allow it to air-dry at room temperature for 48 hours to form hydrogel films.
- Neutralize the films by rinsing with a mild NaOH solution, followed by thorough washing with DI water.
- The dried hydrogel films can be rehydrated before application or applied as a moist dressing directly to the wound.
Validation and Characterization:
- Morphological Analysis (SEM): Image freeze-dried hydrogel samples using Scanning Electron Microscopy to analyze the internal porous structure, pore interconnectivity, and distribution of AgNPs [62].
- Mechanical Testing: Perform tensile tests to determine the ultimate tensile strength, elongation at break, and elastic modulus of the hydrogel films. Compare with control hydrogels without AgNPs to quantify reinforcement.
- Drug Release Kinetics: Immerse the drug-loaded hydrogel in PBS at 37°C under gentle agitation. Withdraw samples at predetermined time points and analyze ciprofloxacin concentration using UV-Vis spectroscopy to generate a release profile [62].
- Anti-Biofilm Assay: Challenge the hydrogel against mature biofilms of multi-drug resistant P. aeruginosa. Use crystal violet staining or colony counting to quantify biofilm biomass reduction compared to untreated controls [62].
Protocol 3: Workflow for In-Silico Design and Experimental Validation
Computational modeling accelerates hydrogel design by predicting structure-property relationships before synthesis. This protocol outlines an integrated in-silico/experimental workflow.
Procedure:
- Define Target Properties: Specify required mechanical (e.g., elastic modulus, toughness) and biological (e.g., degradation rate, drug release profile) parameters for the intended wound healing application [63].
- Molecular Dynamics (MD) Simulation: Use MD software (e.g., GROMACS, LAMMPS) to model polymer chains, crosslinkers, and water molecules at the atomistic/mesoscale. Simulate crosslinking processes and analyze the resulting network topology, chain mobility, and interaction energies to predict mechanical and swelling behavior [63].
- Finite Element Analysis (FEA): Construct a macroscopic model of the hydrogel structure based on MD outputs. Use FEA software (e.g., COMSOL, Abaqus) to simulate the material's response to mechanical stress, fluid flow, and drug diffusion, predicting bulk performance [63].
- Machine Learning (ML) Optimization: Train ML models (e.g., random forest, neural networks) on existing experimental and simulation data. Use the models to identify optimal polymer compositions, crosslinking densities, and fabrication parameters that maximize both mechanical strength and bioactivity [63].
- Experimental Synthesis: Synthesize the top candidate hydrogels identified by the in-silico workflow using the protocols outlined in sections 4.1 and 4.2.
- Characterization and Validation: Characterize the synthesized hydrogels using the techniques listed in Table 3. Compare experimental results with computational predictions.
- Iterative Refinement: Feed experimental results back into the computational models to improve their accuracy and guide the next design iteration, closing the loop between simulation and experiment [63].
The path to advanced wound care hinges on the rational design of hydrogel dressings that do not force a choice between structural integrity and biological function. As detailed in these Application Notes, this balance is achievable through strategic material hybridization, innovative structural engineering (such as dynamic networks and hierarchical ordering), and the integration of bioactive reinforcements. The provided protocols for fabricating self-healing, nanocomposite, and computationally designed hydrogels offer researchers a practical toolkit for developing next-generation wound management solutions. By adopting these integrated approaches, the field can accelerate the creation of truly biomimetic, multifunctional hydrogels that meet the complex demands of healing, particularly for stubborn chronic wounds, ultimately improving patient outcomes and reducing the global healthcare burden.
In the field of wound healing, achieving precise control over the delivery of therapeutic agents is paramount for effectively addressing complex biological processes and pathological barriers. Sustained and targeted drug delivery systems are engineered to overcome the challenges of conventional wound dressings, which often provide uncontrolled drug release, leading to subtherapeutic dosing, systemic toxicity, and inefficient treatment of chronic wounds [67] [68]. Hydrogels, with their highly tunable physicochemical properties and biocompatibility, have emerged as a leading platform for advanced drug delivery in wound care [69] [37]. This document outlines key strategies for controlling drug release from hydrogels, providing detailed application notes and experimental protocols tailored for research on hydrogel fabrication for wound healing. The content is structured to equip researchers and drug development professionals with practical methodologies for developing next-generation wound dressings capable of enhancing therapeutic outcomes through sophisticated release kinetics.
Core Strategies for Controlled Drug Delivery
Advanced hydrogel-based drug delivery systems leverage a combination of material engineering, structural design, and responsive mechanisms to achieve precise control over the release profiles of therapeutic agents. The following core strategies are central to this endeavor, each offering distinct advantages for wound healing applications.
Table 1: Core Strategies for Controlled Drug Delivery in Hydrogels
| Strategy | Mechanism of Action | Key Advantages | Common Materials |
|---|---|---|---|
| Structurally Engineered Release | Controls drug diffusion through engineered pore architecture, multilayered barriers, or matrix density [67]. | Predictable, sustained release; tunable mechanical properties; prevents burst release [67] [70]. | Alginate, Chitosan, Polyvinyl alcohol (PVA), Layer-by-layer polyelectrolytes [67] [37]. |
| Stimuli-Responsive Release | Drug release is triggered by specific pathological or external stimuli in the wound microenvironment [67] [71]. | "On-demand" release; high targeting specificity; maximizes therapeutic efficacy while minimizing side effects [37] [71]. | pH-sensitive polymers (e.g., poly(acrylic acid)), Enzyme-sensitive peptides, Temperature-sensitive polymers (e.g., Poloxamer) [2] [71]. |
| Conjugation & Chemical Binding | Therapeutic agents are covalently conjugated to the hydrogel polymer backbone [68]. | Enhanced drug stability; eliminates initial burst release; release rate tied to hydrogel degradation [68]. | Hyaluronic acid, PEG, functionalized polymers for "click" chemistry [68] [71]. |
| Nanoparticle-Integrated Release | Nanoparticles act as nanoscale reservoirs or carriers within the hydrogel matrix [30] [70]. | Synergistic functions (e.g., antimicrobial, conductive); protects labile drugs; enables multiple release mechanisms [30] [70]. | Silver nanoparticles, Gold nanoparticles, Zinc oxide nanoparticles, Liposomes [30] [70]. |
Application Notes
- Strategy Selection: The choice of strategy depends on the specific wound type and therapeutic goal. For example, chronic wounds with elevated protease levels benefit from enzyme-responsive systems, while infected wounds may require the sustained antimicrobial activity provided by nanoparticle-integrated hydrogels [2] [30].
- Combination Approaches: The most advanced systems often combine multiple strategies. A hydrogel can be structurally designed as a bilayer to offer initial protection, while also incorporating pH-sensitive bonds for triggered release in the acidic environment of a chronic wound [67] [68].
- Material Considerations: Natural polymers like chitosan and hyaluronic acid offer inherent biocompatibility and biodegradability, while synthetic polymers like PVA and PEG provide greater control over mechanical properties and degradation rates [37]. Hybrid systems are increasingly popular to harness the benefits of both [37].
Experimental Protocols
This section provides detailed methodologies for fabricating and characterizing hydrogels employing different controlled-release strategies.
Protocol 1: Fabrication of a Bilayer Structural Hydrogel Dressing
This protocol details the creation of a bilayer hydrogel where a dense, low-porosity layer controls the release of a model hydrophilic drug (e.g., an antibiotic) while a second, high-porosity layer facilitates exudate management [67].
Research Reagent Solutions:
- Solution A (Dense Drug-Loaded Layer): 4% (w/v) Sodium Alginate, 1 mg/mL Model Drug (e.g., Ciprofloxacin), in deionized water.
- Solution B (Porous Absorbent Layer): 2% (w/v) Chitosan in 1% (v/v) acetic acid solution.
- Cross-linking Solution: 2% (w/v) Calcium Chloride (CaClâ‚‚) in deionized water.
Procedure:
Preparation of the Drug-Loaded Layer:
- Dissolve sodium alginate in deionized water under gentle magnetic stirring at 40°C until a clear solution is obtained.
- Allow the solution to cool to room temperature. Add the model drug and stir until fully dissolved and homogenized.
- Pour the solution into a Petri dish to a depth of 1 mm.
- Carefully spray the surface with the 2% CaClâ‚‚ cross-linking solution and incubate for 15 minutes to initiate ionic gelation.
- Remove excess cross-linking solution.
Fabrication of the Porous Layer:
- Dissolve chitosan in the acetic acid solution with stirring until fully dissolved.
- Pour the chitosan solution directly onto the pre-formed, semi-gelled alginate layer to a final combined depth of 3 mm.
- Freeze the entire construct at -20°C for 12 hours, then lyophilize for 24 hours to create a porous structure via the freeze-thawing method [72].
Final Cross-linking and Sterilization:
- Immerse the lyophilized bilayer hydrogel in the 2% CaClâ‚‚ solution for 1 hour to complete the cross-linking of both layers.
- Rinse thoroughly with phosphate-buffered saline (PBS) to neutralize pH and remove unreacted reagents.
- Sterilize under UV light for 30 minutes per side before biological testing.
Diagram 1: Bilayer Hydrogel Fabrication Workflow
Protocol 2: Developing a pH-Responsive Injectable Hydrogel
This protocol describes the synthesis of an injectable, pH-sensitive hydrogel based on carboxymethyl chitosan (CMCS) and alginate-dialdehyde (ADA), which undergoes gelation via dynamic Schiff base formation, offering controlled release in the slightly acidic environment of chronic wounds [68] [71].
Research Reagent Solutions:
- Polymer Solution A: 3% (w/v) Carboxymethyl Chitosan (CMCS) in PBS (pH 7.4).
- Polymer Solution B: 4% (w/v) Alginate-Dialdehyde (ADA) in PBS (pH 7.4). Note: ADA is synthesized by periodate oxidation of sodium alginate.
- Model Drug: A fluorescently tagged protein (e.g., FITC-BSA) to simulate a growth factor.
Procedure:
Polymer Preparation and Drug Loading:
- Dissolve CMCS and ADA in separate vials using PBS as a solvent. Stir until completely clear.
- Add the model drug (FITC-BSA, 0.1 mg/mL) to Polymer Solution B (ADA solution) and mix gently to avoid denaturation.
Hydrogel Formation and Rheology:
- Mix Polymer Solution A and the drug-loaded Polymer Solution B in a 1:1 volume ratio using a dual-barrel syringe and static mixer.
- Immediately transfer the mixture to a rheometer plate. Conduct time-sweep rheometry at 37°C to monitor the storage (G') and loss (G'') moduli over time, confirming gelation when G' surpasses G''.
In Vitro Drug Release Study:
- Inject 1 mL of the pre-gel solution into a release vessel containing 20 mL of release medium (PBS).
- Perform the test at two pH conditions: physiological (pH 7.4) and acidic wound environment (pH 6.0).
- Place the vessel in an incubator shaker at 37°C and 50 rpm.
- At predetermined time intervals, withdraw 1 mL of the release medium and replace it with an equal volume of fresh pre-warmed medium.
- Analyze the drug concentration in the withdrawn samples using a fluorescence plate reader or HPLC. Plot the cumulative release percentage over time to characterize the pH-responsive profile.
Table 2: Expected Cumulative Drug Release (%) Over Time from pH-Responsive Hydrogel
| Time (Hours) | pH 7.4 | pH 6.0 |
|---|---|---|
| 2 | 15 ± 3% | 25 ± 4% |
| 8 | 30 ± 4% | 55 ± 5% |
| 24 | 45 ± 5% | 80 ± 6% |
| 48 | 60 ± 5% | 95 ± 3% |
The Scientist's Toolkit: Essential Research Reagents
Table 3: Key Reagents for Hydrogel-Based Drug Delivery Research
| Reagent/Material | Function/Application | Key Characteristics |
|---|---|---|
| Natural Polymers (Alginate, Chitosan, Hyaluronic Acid) | Base materials for hydrogel formation; provide biocompatibility and biodegradability [8] [37]. | Often require ionic or chemical cross-linking; inherently bioactive. |
| Synthetic Polymers (PEG, PVA, Pluronic F127) | Offer precise control over mechanical properties and degradation kinetics; used for creating tunable networks [69] [37]. | Defined molecular weight; can be functionalized for "click" chemistry. |
| Genipin | Natural cross-linking agent as a safer alternative to glutaraldehyde [30]. | Forms stable, fluorescent blue pigments; low cytotoxicity. |
| Methacrylated Polymers (e.g., GelMA) | Enable photo-polymerization for spatial control over hydrogel formation [71]. | Cures under UV/visible light in the presence of a photoinitiator. |
| N,N'-Methylenebisacrylamide (MBA) | Chemical cross-linker for synthetic hydrogels like polyacrylamide [30]. | Creates covalent amide bonds between polymer chains. |
| Silver Nanoparticles (AgNPs) | Multifunctional nanomaterial providing sustained antimicrobial activity within hydrogels [30] [70]. | Broad-spectrum antimicrobial; can be synthesized via green methods. |
| Latanoprost ethyl amide-d4 | Latanoprost Ethyl Amide-d4 | Latanoprost ethyl amide-d4 is an internal standard for GC/LC-MS. For Research Use Only. Not for human or veterinary diagnostic or therapeutic use. |
| Nucleoprotein (118-126) | Nucleoprotein (118-126), MF:C43H69N13O13S, MW:1008.2 g/mol | Chemical Reagent |
Advanced Systems and Characterization
Nanoparticle-Integrated Hydrogel Systems
The integration of nanoparticles into hydrogels represents a paradigm shift towards multifunctional dressings. Nanoparticles can be pre-loaded with drugs and then incorporated into the hydrogel, acting as secondary reservoirs for sustained release or enabling triggered release in response to external stimuli like near-infrared light [30] [70].
Protocol 3: Incorporating Drug-Loaded Liposomes into a GelMA Hydrogel
This protocol creates a multi-stage release system, ideal for delivering multiple growth factors at different phases of wound healing [71].
Procedure:
- Liposome Preparation: Prepare drug-loaded liposomes (e.g., containing Vancomycin) using a thin-film hydration and extrusion method.
- Hydrogel Composite Formation: Resuspend the purified, drug-loaded liposomes in a GelMA solution containing a photoinitiator (e.g., LAP) and a second water-soluble drug (e.g., Deferoxamine).
- Photo-Cross-linking: Expose the mixture to UV light (365 nm, 5 mW/cm²) for 60 seconds to form a stable composite hydrogel.
- Characterization: Use electron microscopy to confirm liposome distribution within the hydrogel network. The release profile will show an initial burst of the freely soluble drug (Deferoxamine) followed by a sustained release of the liposome-encapsulated drug (Vancomycin) as the liposomes degrade.
Diagram 2: Nanoparticle-Hydrogel Composite Fabrication
Critical Characterization Techniques
Rigorous characterization is essential to correlate hydrogel design with its performance.
- Swelling Ratio: Measure the mass of the dry hydrogel (Wâ‚€), then incubate in PBS (pH 7.4) until equilibrium swelling. Remove surface water and weigh the swollen hydrogel (Wâ‚›). The swelling ratio is (Wâ‚› - Wâ‚€)/Wâ‚€. High swelling can indicate a more open network for drug diffusion [37].
- Drug Release Kinetics: Conduct in vitro release studies as described in Protocol 2. Fit the release data to mathematical models (e.g., Higuchi, Korsmeyer-Peppas) to determine the primary release mechanism (e.g., Fickian diffusion, polymer relaxation) [68].
- Mechanical Testing: Perform compression or tensile testing to determine the elastic modulus (Young's modulus), which should ideally match that of native skin (~0.5 - 2 MPa) to provide mechanical support and integrate properly with the wound bed [69] [37].
- Biocompatibility and Efficacy: Always follow in vitro release studies with cell viability assays (e.g., with fibroblasts like L929 or NIH/3T3) and subsequent in vivo studies in established wound models (e.g., diabetic mouse full-thickness wound model) to validate safety and healing efficacy [72] [8].
The increasing prevalence of microbial infections, particularly those associated with impaired wound healing and biomedical implant failure, has spurred significant development of advanced antimicrobial materials [73]. Among these, hydrogels—three-dimensional, hydrophilic polymer networks capable of absorbing large amounts of water—have emerged as particularly promising platforms for antimicrobial therapy [73] [74]. Their structural similarity to the native extracellular matrix (ECM), high biocompatibility, and tunable physical and chemical properties make them ideal for wound dressing applications and as coatings for medical implants [18] [75].
The threat of antimicrobial resistance (AMR) underscores the critical need for innovative solutions. It is estimated that drug-resistant bacteria could cause millions of deaths annually worldwide, highlighting the urgency for developing new antibacterial strategies beyond conventional antibiotics [74]. Antimicrobial hydrogels address this challenge through two primary design philosophies: intrinsic antimicrobial activity, where the hydrogel material itself possesses properties that inhibit microbes, and loaded antimicrobial systems, where the hydrogel acts as a reservoir for the controlled release of antimicrobial agents [73] [76]. This application note details the design principles, fabrication protocols, and evaluation methods for both intrinsic and agent-loaded antimicrobial hydrogels, providing a framework for their development within wound healing research.
Hydrogel Design Strategies and Antimicrobial Mechanisms
Intrinsically Antimicrobial Hydrogels
Intrinsically antimicrobial hydrogels are fabricated from polymers that possess inherent microbial inhibition properties, eliminating the need for additional bioactive compounds.
Table 1: Characteristics of Key Intrinsically Antimicrobial Polymers
| Polymer | Source | Primary Antimicrobial Mechanism | Key Factors Influencing Activity |
|---|---|---|---|
| Chitosan [76] | Deacetylated chitin (crustacean shells) | Electrostatic interaction between cationic amine groups and negatively charged microbial membranes, causing membrane disruption and leakage [76]. | Degree of deacetylation, molecular weight, pH, polymer concentration [76]. |
| Antimicrobial Peptides (AMPs) [73] [76] | Natural or synthetic polypeptides | Membrane disruption via pore formation or carpet model; some have intracellular targets [76]. | Amino acid sequence, chain length, amphipathicity [76]. |
| Cationic Polymers [73] | Synthetic (e.g., polycations) | Surface contact killing; cationic groups disrupt bacterial membranes [73]. | Charge density, polymer architecture, hydrophobicity [73]. |
The following diagram illustrates the primary mechanism of action for cationic intrinsically antimicrobial hydrogels like chitosan.
Hydrogels with Loaded Antimicrobial Agents
An alternative strategy involves loading pre-formed hydrogels with antimicrobial agents that are released in a controlled manner to combat infection. This approach offers versatility by combining the biocompatible scaffold of the hydrogel with the potent activity of various antimicrobials.
Table 2: Overview of Loaded Antimicrobial Agents for Hydrogels
| Loaded Agent | Class | Antimicrobial Mechanism | Key Advantages |
|---|---|---|---|
| Silver Nanoparticles (AgNPs) [73] [62] | Metal Nanoparticle | Generation of reactive oxygen species (ROS); damage to cell membranes; inhibition of enzymatic activity [73]. | Broad-spectrum activity, low propensity for bacterial resistance [73]. |
| Gold Nanoparticles (AuNPs) [73] | Metal Nanoparticle | Antibacterial properties, often enhanced in bimetallic systems with silver [73]. | Tunable properties, potential for photothermal therapy [73]. |
| Ciprofloxacin [73] [74] [62] | Antibiotic (Fluoroquinolone) | Inhibition of bacterial DNA gyrase and topoisomerase IV, blocking DNA replication [74]. | Broad-spectrum activity, well-characterized efficacy [62]. |
| Nitric Oxide (NO) [62] | Gaseous Therapeutic | Multiple metabolic mechanisms, including nitrosative stress, that constrain bacterial resistance development [62]. | Broad-spectrum antibacterial and pro-healing effects [62]. |
The general workflow for creating and evaluating a loaded antimicrobial hydrogel is summarized below.
Experimental Protocols
Protocol 1: Fabrication of an Intrinsically Antimicrobial Chitosan-Gelatin-Metal Ion Hydrogel
This protocol describes the synthesis of a porous chitosan-based hydrogel crosslinked with gelatin and metal ions (Ag+, Cu2+, Zn2+), demonstrating significant antibacterial activity and high biocompatibility [77].
Materials and Reagents
- Chitosan (CS) (Medium molecular weight, deacetylation degree >75%)
- Gelatin (GEL) (Type A, from porcine skin)
- Formaldehyde (37% solution)
- Metal Salts: Silver nitrate (AgNO3), Copper sulfate (CuSO4), Zinc acetate (Zn(CH3COO)2)
- Acetic Acid (3% v/v aqueous solution)
- Deionized Water
Procedure
- Prepare Chitosan Solution: Dissolve 2 g of chitosan in 100 mL of 3% acetic acid solution with continuous stirring for 2 hours at room temperature until a clear, viscous solution is obtained.
- Chemical Activation: Add 1 mL of formaldehyde (37%) to the chitosan solution and stir for 30 minutes to activate the amino groups.
- Introduce Gelatin: Add 1 g of gelatin to the activated chitosan solution. Stir the mixture at 40°C for 1 hour to facilitate covalent attachment and physical interaction between CS and GEL.
- Form Hydrogel with Metal Ions:
- Prepare separate 0.1 M aqueous solutions of AgNO3, CuSO4, and Zn(CH3COO)2.
- Add the metallic salt solution dropwise to the CS-GEL mixture under vigorous stirring.
- Observe the formation of a stable, elastic hydrogel. Typical gelation times are ~3 hours for Ag+, ~6 hours for Cu2+, and ~5 hours for Zn2+ [77].
- Purification: Wash the formed hydrogels thoroughly with deionized water to remove any unreacted precursors.
- Drying: Lyophilize the purified hydrogels for 48 hours to obtain a porous scaffold for further characterization and use.
Characterization and Evaluation
- Fourier-Transform Infrared (FTIR) Spectroscopy: Confirm metal ion binding to amino and hydroxyl groups on chitosan.
- Field Emission Scanning Electron Microscopy (FESEM): Analyze the crosslinked, porous structure of the freeze-dried hydrogels.
- Rheological Tests: Measure the mechanical strength and gelation kinetics.
- Antibacterial Assay: Evaluate efficacy against S. aureus and E. coli using agar well diffusion and broth dilution methods to determine Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC).
- Biocompatibility Test: Assess cytotoxicity using an MTT assay on human fibroblast (HFF-1) cells over 48 hours.
Protocol 2: Fabrication of a Multi-Antimicrobial Loaded Hydrogel (Hy-NO-Ag-Cip)
This protocol outlines the synthesis of a chitosan-based hydrogel matrix co-loaded with nitric oxide (NO), silver nanoparticles (AgNPs), and the antibiotic ciprofloxacin (Cip) for enhanced antibacterial and wound healing properties against multi-drug-resistant pathogens [62].
Materials and Reagents
- Chitosan (Low molecular weight)
- S-Nitrosoglutathione (GSNO) (as NO donor)
- Poly(vinyl methyl ether co-maleic anhydride) (PVMMA)
- Silver Nanoparticles (AgNPs) (~20 nm, commercially sourced or synthesized)
- Ciprofloxacin hydrochloride
- Acetic Acid (3% v/v aqueous solution)
- Phosphate Buffered Saline (PBS), pH 7.4
Procedure
- Prepare Chitosan Solution: Dissolve 2 g of chitosan in 100 mL of 3% acetic acid solution with continuous stirring for 2 hours.
- Prepare GSNO-PVMMA-Drug Solution: Dissolve 5 g of GSNO-PVMMA, 50 mg of AgNPs, and 50 mg of ciprofloxacin in 100 mL of distilled water. Stir steadily at 50°C for 4 hours.
- Combine Solutions: Mix the chitosan and GSNO-PVMMA-drug solutions in a 4:1 ratio (v/v) using mechanical blending at 1000 rpm for 10 minutes.
- Casting and Drying: Pour the final mixture onto Pyrex petri dishes and allow it to air-dry at room temperature for two days.
- Storage: Store the dried hydrogel films in a desiccator protected from light until use.
Characterization and Evaluation
- SEM: Visualize the ultrastructure and interconnected porous network.
- FTIR: Confirm the successful incorporation of all components.
- Swelling Studies: Determine the Equilibrium Swelling Ratio (ESR) in PBS at different temperatures using the formula: ( \text{ESR} = ww / wd ), where ( ww ) and ( wd ) are the weights of the swelled and dried hydrogel, respectively [62].
- Drug Release Kinetics: Use a UV spectrophotometer to quantify the release profile of ciprofloxacin and Ag+ ions into PBS over time.
- In vitro Antibiofilm Assay: Assess the ability of the hydrogel to disrupt and inhibit mature biofilms of target bacteria (e.g., P. aeruginosa).
- In vivo Wound Healing: Induce burn wound infections in an animal model (e.g., rats) and topically apply the hydrogel to monitor wound closure, bacterial load, and histological parameters like epithelial thickness.
The Scientist's Toolkit: Essential Research Reagents and Materials
Table 3: Key Research Reagent Solutions for Antimicrobial Hydrogel Development
| Reagent/Material | Function | Example Application & Notes |
|---|---|---|
| Chitosan [76] [77] | Base polymer providing intrinsic antimicrobial activity and forming the hydrogel scaffold. | Use varying molecular weights and degrees of deacetylation to tune mechanical properties and antimicrobial efficacy [76]. |
| Gelatin [78] [77] | Natural polymer used as a biocompatible crosslinker; promotes cell adhesion via RGD sequences. | Crosslinks with chitosan via Schiff base formation; enhances the biocompatibility of the final construct [77]. |
| Genipin [74] | Natural, low-toxicity crosslinking agent as an alternative to glutaraldehyde. | Crosslinks amine-containing polymers (e.g., chitosan, gelatin); results in blue pigmentation [74]. |
| Silver Nitrate (AgNO3) [73] [77] | Precursor for forming silver nanoparticles (AgNPs) within the hydrogel or as a source of Ag+ ions. | Can be reduced in situ to form AgNPs; provides broad-spectrum antimicrobial activity [73] [77]. |
| Ciprofloxacin HCl [62] | Broad-spectrum antibiotic for loading into hydrogels. | Effective against common skin pathogens; used for local administration to minimize systemic side effects [62]. |
| S-Nitrosoglutathione (GSNO) [62] | Nitric oxide (NO) donor molecule. | Provides controlled release of NO, imparting antibacterial and pro-healing properties [62]. |
The strategic design of hydrogels with either intrinsic or loaded antimicrobial properties offers powerful tools to combat infection in wound care. Intrinsic systems, such as those based on chitosan, provide a fundamental level of microbial resistance without relying on the release of agents, potentially reducing concerns about depletion over time [76] [77]. Conversely, loaded systems offer high versatility and potency by delivering a wide range of antimicrobials—from traditional antibiotics and metal nanoparticles to gaseous mediators like nitric oxide—directly to the infection site in a controlled manner [73] [62].
The future of antimicrobial hydrogels lies in the development of multifunctional and "smart" systems that can respond to specific wound microenvironment triggers (e.g., pH, enzymes, bacterial toxins) to release antimicrobials on demand [1] [54]. Combining multiple antimicrobial strategies, such as the concurrent use of AgNPs, NO, and antibiotics, can yield synergistic effects and reduce the likelihood of resistance development [62]. As research progresses, the focus must remain on balancing potent antimicrobial activity with excellent biocompatibility and mechanical performance to meet the complex demands of advanced wound healing applications.
Ensuring Biosafety and Managing Degradation By-Products
The advancement of hydrogel-based wound dressings represents a paradigm shift in regenerative medicine, offering dynamic solutions for chronic wound management. However, the clinical translation of these innovative biomaterials necessitates rigorous assessment of their biosafety profile and a comprehensive understanding of their degradation behavior. Hydrogels, three-dimensional hydrophilic polymer networks capable of absorbing significant amounts of water, must demonstrate exceptional biocompatibility and predictable degradation kinetics to ensure successful integration with host tissue without eliciting adverse immune responses [79] [33]. The degradation process of hydrogels generates various by-products whose accumulation could potentially trigger cytotoxicity, immunogenicity, or other unintended biological consequences [37]. This application note provides detailed protocols for evaluating hydrogel biosafety and characterizing degradation by-products within the context of wound healing applications, specifically focusing on standardized testing methodologies and analytical techniques that meet regulatory requirements for biomedical devices.
Biosafety Assessment of Hydrogel Formulations
Cytocompatibility Testing Protocols
Direct Contact Cytotoxicity Assay (ISO 10993-5) This protocol evaluates the potential cytotoxic effects of hydrogel extracts or direct material contact on mammalian cell lines, providing crucial data on material safety before in vivo applications.
Materials Required:
- Test Hydrogel: Sterile, fully crosslinked hydrogel samples (1 cm² surface area, 2 mm thickness)
- Cell Line: L929 mouse fibroblast cells (ATCC CCL-1) or human dermal fibroblasts (ATCC PCS-201-012)
- Culture Medium: Dulbecco's Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin
- Extraction Vehicle: Serum-free DMEM medium
- Positive Control: Latex extract or polyurethane containing 0.1% zinc diethyldithiocarbamate
- Negative Control: High-density polyethylene (RM-A) or titanium medical grade extract
- Detection Reagent: MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) solution (5 mg/mL in PBS)
Experimental Procedure:
- Sample Preparation: Using aseptic technique, prepare triplicate hydrogel samples under laminar flow hood. For extract testing, place samples in extraction vehicle at 3 cm²/mL surface-area-to-volume ratio. Incubate at 37°C for 24±2 hours with occasional agitation.
- Cell Seeding: Seed L929 fibroblasts in 96-well plates at density of 1×10ⴠcells/well in 100 μL complete medium. Incubate for 24±1 hours at 37°C in 5% CO₂ to form near-confluent monolayers.
- Exposure: Remove culture medium and replace with either:
- 100 μL of hydrogel extract
- Direct contact with sterile hydrogel samples placed on cell monolayer Include positive, negative, and blank (medium only) controls
- Incubation: Incubate plates for 24±2 hours at 37°C in 5% CO₂
- Viability Assessment: Remove test materials/extracts and add 100 μL MTT solution (0.5 mg/mL final concentration). Incubate for 2-4 hours until purple formazan crystals are visible.
- Solubilization: Carefully remove MTT solution and add 100 μL dimethyl sulfoxide (DMSO) to solubilize formazan crystals. Agitate plates gently for 10 minutes.
- Measurement: Measure absorbance at 570 nm with reference wavelength of 650 nm using microplate reader.
- Calculation: Calculate percentage cell viability relative to negative control:
Cell Viability (%) = (Absorbance of test sample / Absorbance of negative control) × 100
Interpretation: Hydrogel formulations demonstrating ≥70% cell viability relative to negative control are considered non-cytotoxic according to ISO 10993-5 standards [80] [37].
Hemocompatibility Assessment
Hemolysis Assay for Blood-Contacting Applications This protocol evaluates the potential of hydrogel materials to cause erythrocyte damage and hemoglobin release, particularly relevant for wound dressings that may contact blood.
Materials Required:
- Anticoagulated Whole Blood: Fresh human or animal blood collected in sodium citrate (3.8%)
- Dilution Medium: 0.9% sterile saline solution
- Positive Control: Distilled water
- Negative Control: 0.9% saline solution
Experimental Procedure:
- Sample Preparation: Incubate sterile hydrogel samples (1 cm²) in 10 mL saline at 37°C for 30 minutes
- Blood Dilution: Dilute fresh anticoagulated blood 1:10 with saline
- Exposure: Add 0.2 mL diluted blood to each pre-incubated sample tube. Include positive (water) and negative (saline) controls
- Incubation: Gently mix and incubate at 37°C for 60±5 minutes
- Centrifugation: Centrifuge all tubes at 800× g for 15 minutes
- Measurement: Transfer supernatants to clean tubes and measure absorbance at 545 nm
- Calculation: Calculate percentage hemolysis using formula:
Hemolysis (%) = [(Absorbance of test sample - Absorbance of negative control) / (Absorbance of positive control - Absorbance of negative control)] × 100
Interpretation: Hydrogel materials demonstrating <5% hemolysis are considered non-hemolytic, while >5% indicates potential blood compatibility issues [80].
Table 1: Biosafety Assessment Criteria for Hydrogel Wound Dressings
| Test Parameter | Standard Method | Acceptance Criteria | Frequency |
|---|---|---|---|
| Cytotoxicity | ISO 10993-5 | ≥70% cell viability | Each batch |
| Hemocompatibility | ASTM F756 | <5% hemolysis | Each formulation |
| Pyrogenicity | Bacterial Endotoxin Test | <0.5 EU/mL | Each sterilization cycle |
| Sensitization | ISO 10993-10 | No sensitization response | Each material change |
| Intracutaneous Reactivity | ISO 10993-10 | Mean score ≤1.0 | Each material change |
| Acute Systemic Toxicity | ISO 10993-11 | No biological reactivity | Each material family |
Characterization of Hydrogel Degradation
In Vitro Degradation Monitoring
Gravimetric Analysis of Degradation Kinetics This protocol quantitatively monitors hydrogel mass loss over time under simulated wound fluid conditions, providing critical data on material residence time and structural integrity.
Materials Required:
- Simulated Wound Fluid (SWF): 1× PBS containing 100 U/mL collagenase Type I or 2 U/mL lysozyme, pH 7.4±0.2
- Analytical Balance: Precision ±0.01 mg
- Drying Oven: Maintained at 37±1°C
- Freeze Dryer: For lyophilization of hydrogel samples
Experimental Procedure:
- Baseline Measurement:
- Pre-weigh empty 24-well plate (Wplate)
- Add 1 mL SWF to each well
- Carefully place pre-hydrated hydrogel samples (approximately 10×10×2 mm) into each well
- Weigh plate with samples and medium (Winitial)
- Calculate initial hydrogel mass: Minitial = Winitial - Wplate - Mmedium
Degradation Protocol:
- Incubate plates at 37°C under gentle agitation (50 rpm)
- Replace SWF every 48 hours to maintain enzyme activity
- At predetermined time points (days 1, 3, 7, 14, 21, 28), carefully remove samples
- Rinse with deionized water and freeze-dry for 24 hours
- Weigh dried samples (Mdry)
Calculation: Calculate remaining mass percentage at each time point:
Remaining Mass (%) = (Mdry / Minitial) × 100
- Degradation Rate Constant: Plot natural logarithm of remaining mass versus time. Slope represents degradation rate constant (k):
ln(Mt/M0) = -kt
Interpretation: Degradation profiles should correlate with wound healing timelines (typically 2-4 weeks for chronic wounds). Rapid degradation may necessitate crosslinking optimization, while slow degradation might require enzymatic modification [33] [37].
By-Product Analysis
Chromatographic Characterization of Degradation Products This protocol identifies and quantifies specific degradation by-products released from hydrogels during degradation, assessing potential bioaccumulation risks.
Materials Required:
- UPLC-MS System: Ultra-performance liquid chromatography coupled with mass spectrometry
- Analytical Columns: C18 reverse-phase column (2.1×100 mm, 1.7 μm)
- Mobile Phase:
- Phase A: 0.1% formic acid in water
- Phase B: 0.1% formic acid in acetonitrile
- Standards: Reference compounds for expected degradation products
Experimental Procedure:
- Sample Collection: Collect degradation medium at predetermined time points throughout degradation study
- Sample Preparation: Filter samples through 0.22 μm membrane, dilute 1:10 with mobile phase A
- UPLC Conditions:
- Flow rate: 0.3 mL/min
- Column temperature: 40°C
- Injection volume: 5 μL
- Gradient program: 5% B to 95% B over 15 minutes
- MS Detection:
- Ionization mode: Electrospray ionization (ESI) positive/negative switching
- Mass range: m/z 50-2000
- Source temperature: 150°C
- Desolvation temperature: 500°C
- Data Analysis: Identify degradation products by comparing retention times and mass spectra with reference standards. Quantify using external calibration curves.
Interpretation: Monitor for potentially cytotoxic monomers, crosslinkers, or oligomeric fragments. For natural hydrogels (alginate, chitosan, collagen), monitor sugar monomers and amino acids. For synthetic hydrogels (PEG, PAA), monitor polymer fragments and crosslinker residues [37].
Table 2: Analytical Techniques for Degradation By-Product Characterization
| Technique | Application | Detection Limit | Key Parameters |
|---|---|---|---|
| Size Exclusion Chromatography | Molecular weight distribution | 1 μg/mL | Mn, Mw, PDI changes |
| Nuclear Magnetic Resonance | Structural identification of fragments | 10 μM | Chemical shift analysis |
| Fourier Transform Infrared Spectroscopy | Functional group changes | 1% composition | Carbonyl, amine peaks |
| Liquid Chromatography-Mass Spectrometry | Quantitative by-product analysis | 0.1 ng/mL | Fragment identification |
| Enzyme-Linked Immunosorbent Assay | Inflammatory cytokine detection | 1 pg/mL | TNF-α, IL-6, IL-1β |
Advanced Biosafety Evaluation
Immunomodulatory Response Profiling
Macrophage Polarization Assay This protocol evaluates the immunomodulatory effects of hydrogel degradation products on macrophage function, particularly relevant for chronic wounds where prolonged inflammation impedes healing.
Materials Required:
- Cell Line: RAW 264.7 murine macrophages or THP-1 human monocyte cell line
- Differentiation Agents: Phorbol 12-myristate 13-acetate (PMA) for THP-1 differentiation
- Polarization Inducers:
- M1 polarization: LPS (100 ng/mL) + IFN-γ (20 ng/mL)
- M2 polarization: IL-4 (20 ng/mL)
- Detection Antibodies: Anti-CD86 (M1 marker), Anti-CD206 (M2 marker)
- Cytokine ELISA Kits: TNF-α, IL-10, IL-12, TGF-β
Experimental Procedure:
- Macrophage Culture: Maintain RAW 264.7 cells in RPMI-1640 with 10% FBS. For THP-1 cells, differentiate with 100 nM PMA for 48 hours
- Hydrogel Extract Preparation: Prepare extracts as described in Section 2.1 using serum-free medium
- Exposure and Polarization:
- Seed macrophages in 12-well plates (5×10ⵠcells/well)
- Pre-treat with hydrogel extracts for 24 hours
- Induce polarization with specific cytokines for additional 24 hours
- Flow Cytometry Analysis:
- Harvest cells and stain with fluorochrome-conjugated anti-CD86 and anti-CD206 antibodies
- Analyze using flow cytometer, collecting ≥10,000 events per sample
- Calculate M1/M2 ratio based on surface marker expression
- Cytokine Profiling:
- Collect culture supernatants after polarization
- Measure pro-inflammatory (TNF-α, IL-12) and anti-inflammatory (IL-10, TGF-β) cytokines using ELISA
- Statistical Analysis: Perform one-way ANOVA with post-hoc Tukey test, considering p<0.05 statistically significant
Interpretation: Ideal wound healing hydrogels should promote transition from pro-inflammatory M1 to anti-inflammatory M2 phenotype. Significant increase in M1 markers or pro-inflammatory cytokines may indicate immunogenicity concerns [80].
The following diagram illustrates the experimental workflow for comprehensive hydrogel biosafety and degradation assessment:
Experimental Workflow for Hydrogel Biosafety and Degradation Assessment
In Vivo Biocompatibility Evaluation
Subcutaneous Implantation Model (ISO 10993-6) This protocol assesses local tissue responses to hydrogel materials and their degradation products in a physiologically relevant environment.
Materials Required:
- Animal Model: Sprague-Dawley rats (250-300 g) or BALB/c mice (20-25 g)
- Surgical Materials: Sterile surgical instruments, absorbable sutures, anesthesia equipment
- Histology Supplies: 10% neutral buffered formalin, paraffin embedding materials, hematoxylin and eosin (H&E) stain
Experimental Procedure:
- Sample Preparation: Sterilize hydrogel samples (10×10×2 mm) by ethylene oxide or gamma irradiation
- Surgical Implantation:
- Anesthetize animals following institutional guidelines
- Make bilateral dorsal incisions and create subcutaneous pockets
- Implant test materials and appropriate controls (negative: polyethylene, positive: latex)
- Close incisions with sutures
- Study Duration: Euthanize animals at 1, 4, and 12 weeks post-implantation (n=6 per time point)
- Histopathological Evaluation:
- Excise implantation sites with surrounding tissue
- Fix in 10% neutral buffered formalin for 24-48 hours
- Process through graded ethanol series, embed in paraffin
- Section at 5 μm thickness and stain with H&E
- Scoring System: Evaluate tissue response using standardized scoring for:
- Polymorphonuclear leukocytes (acute inflammation)
- Lymphocytes (chronic inflammation)
- Plasma cells (immune response)
- Macrophages (phagocytic activity)
- Giant cells (foreign body reaction)
- Fibrosis (encapsulation)
Interpretation: Compare test article responses to controls. Successful hydrogels demonstrate minimal to mild inflammation that decreases over time, with formation of organized fibrous capsule without excessive fibrosis [33].
The Scientist's Toolkit: Research Reagent Solutions
Table 3: Essential Materials for Hydrogel Biosafety and Degradation Studies
| Category | Specific Reagents | Function | Examples & Alternatives |
|---|---|---|---|
| Cell Culture Systems | L929 fibroblasts, Human dermal fibroblasts, RAW 264.7 macrophages | Cytotoxicity and immunomodulation assessment | ATCC CCL-1, ATCC PCS-201-012, ATCC TIB-71 |
| Molecular Detection | ELISA kits (TNF-α, IL-6, IL-10), MTT reagent, Live/Dead assay | Quantification of immune response and cell viability | R&D Systems DuoSet ELISA, Thermo Fisher MTT kit, Calcein-AM/EthD-1 |
| Degradation Media | Collagenase Type I, Lysozyme, Hyaluronidase | Simulation of enzymatic wound environment | Worthington CLS-1, Sigma L4919, STEMCELL 07493 |
| Analytical Standards | Poly(ethylene glycol) oligomers, Acrylic acid, Lactic acid | Quantification of degradation by-products | Sigma 81210, Sigma 147230, Sigma 69775 |
| Histology Reagents | Hematoxylin, Eosin, Masson's Trichrome stain | Tissue response evaluation | Sigma GHS316, Sigma 318906, Sigma HT15 |
| Animal Models | Sprague-Dawley rats, C57BL/6 mice, Diabetic db/db mice | In vivo biocompatibility and wound healing | Charles River Laboratories, The Jackson Laboratory |
| Quality Controls | Latex extracts, Polyethylene, Titanium discs | Reference materials for standardized testing | Hatano Research Institute RM-A, RM-C |
Ensuring biosafety and effectively managing degradation by-products represents a critical pathway to clinical translation for hydrogel-based wound healing technologies. The comprehensive protocols outlined in this application note provide researchers with standardized methodologies for evaluating these essential parameters, from initial cytocompatibility screening to sophisticated immunomodulatory profiling. By integrating these assessment strategies early in the development pipeline, researchers can identify potential safety concerns, optimize material formulations, and ultimately accelerate the development of next-generation hydrogel dressings that offer both therapeutic efficacy and demonstrated safety profiles. The experimental workflows and analytical techniques detailed herein establish a robust framework for hydrogel characterization that aligns with regulatory requirements while advancing the fundamental understanding of material-biology interactions in wound healing applications.
The transition of hydrogels from promising laboratory innovations to clinically reliable and commercially viable products for wound healing is contingent upon overcoming significant scaling, reproducibility, and manufacturing challenges. While the exceptional biocompatibility, moisture retention, and drug-delivery capabilities of hydrogels are well-documented in research settings, the path to industrial-scale production reveals a complex landscape of technical obstacles [33] [8]. These hurdles, including batch-to-batch variability, stringent sterilization requirements, and the integration of sensitive bioactive cargo, often impede clinical translation and consistent product performance [81]. This Application Note details these specific challenges and provides standardized protocols and analytical methodologies designed to enhance reproducibility and streamline the scaling process for hydrogel-based wound healing applications.
Quantitative Analysis of Scaling Challenges
A critical step in addressing scaling hurdles is their quantitative identification and monitoring. The following parameters are essential for assessing production consistency across different scales.
Table 1: Key Quantitative Parameters for Monitoring Hydrogel Scaling Challenges
| Parameter | Laboratory Scale (Bench) | Pilot Scale | Industrial Scale | Impact on Final Product |
|---|---|---|---|---|
| Batch-to-Batch Consistency | High variability (≥15% in properties) [81] | Target <10% variability | Target <5% variability | Affects therapeutic efficacy and release kinetics [81] |
| Gelation Time | Manually tuned; high sensitivity to ambient conditions | Requires automated control systems | Precise, reproducible control critical | Determines injectability and in situ stability [33] [81] |
| Mechanical Strength (Compressive Modulus) | Varies with manual mixing and crosslinking | Varies with scaling of mixing efficiency | Must meet narrow specifications | Impacts scaffold integrity and cell migration in wound bed [33] [81] |
| Sterilization Failure Rate | Low (e.g., filtration) | N/A | High cost of failure for terminal methods (e.g., radiation, heat) [81] | Can alter hydrogel network structure and bioactivity [81] |
| Drug/Cell Loading Efficiency | High control in small batches | Challenges in homogeneous distribution | Efficiency drops due to mixing limitations | Directly influences dosage and therapeutic outcome [81] |
Detailed Experimental Protocols
Protocol for Assessing Batch-to-Batch Reproducibility
This protocol establishes a standard workflow for quantifying and minimizing variability in key hydrogel properties during scale-up.
3.1.1. Objective: To ensure consistent mechanical properties, swelling behavior, and gelation kinetics across multiple production batches during scaling.
3.1.2. Materials:
- Precursor polymers (e.g., Sodium Alginate, Chitosan, PEG-based resins)
- Crosslinking agents (e.g., CaClâ‚‚, Genipin, APS-TEMED for radical polymerization)
- Purified Water (WFI quality recommended)
- Rheometer (e.g., rotational or oscillatory)
- Mechanical tester (e.g., texture analyzer or dynamic mechanical analyzer)
- Lyophilizer
3.1.3. Methodology:
- Standardized Pre-conditioning: Establish and strictly adhere to a standard hydration protocol for all raw materials (e.g., 24 hours at 4°C in a controlled humidity chamber).
- Controlled Synthesis:
- Lab Scale: Utilize magnetic stirring at a fixed RPM (e.g., 500 RPM) for a defined duration.
- Pilot/Industrial Scale: Employ overhead stirrers with geometrically similar impellers. Record power consumption and torque as indirect measures of viscosity development.
- Crosslinking: Implement a controlled addition method for crosslinkers (e.g., syringe pump at lab scale, calibrated metering pump at large scale). Monitor temperature throughout the reaction.
- Post-processing: Standardize purification (e.g., dialysis duration, water change cycles) and lyophilization cycles (ramp rates, final temperature, pressure) across all batches.
3.1.4. Data Collection and Analysis:
- Gelation Kinetics: Use a rheometer with a time-sweep program to monitor the storage (G') and loss (G") moduli. Record the gelation point (crossover of G' and G") and final modulus for each batch.
- Swelling Ratio (Q): Weigh lyophilized hydrogel samples (Wd), immerse in PBS (pH 7.4) at 37°C for 24 hours, then re-weigh after removing surface water (Ws). Calculate Q = (Ws - Wd) / Wd. Report mean and standard deviation across n=5 samples per batch.
- Mechanical Testing: Perform unconfined compression tests on swollen hydrogel discs to determine the compressive modulus. Report mean and standard deviation across n=5 samples per batch.
3.1.5. Troubleshooting:
- High variability in gelation time: Check crosslinker concentration accuracy and environmental temperature control.
- Inconsistent mechanical properties: Verify mixing efficiency and ensure complete dissolution of polymer precursors before crosslinking.
Diagram 1: Workflow for assessing batch-to-batch reproducibility.
Protocol for Scaling Crosslinking Strategies
The crosslinking method is a primary determinant of hydrogel properties. This protocol compares common techniques for scalability.
3.2.1. Objective: To evaluate and optimize different crosslinking strategies (ionic, chemical, enzymatic) for their suitability in large-scale hydrogel production.
3.2.2. Materials:
- Polymer solution (e.g., 2% w/v Sodium Alginate; 1.5% w/v Chitosan in dilute acetic acid)
- Ionic crosslinker (e.g., 100 mM CaClâ‚‚ solution)
- Chemical crosslinker (e.g., 0.5% w/v Genipin solution)
- Enzyme crosslinker (e.g., Microbial Transglutaminase, MTGase)
- Mixing apparatus (magnetic stirrer, overhead stirrer)
- UV-Vis Spectrophotometer (for Genipin crosslinking monitoring)
3.2.3. Methodology:
- Ionic Crosslinking (e.g., Alginate-CaClâ‚‚):
- Lab Scale: Add alginate solution dropwise into a CaClâ‚‚ bath under gentle stirring.
- Scale-up Challenge: Droplet formation is not feasible at large scale.
- Scale-up Solution: Use an internal setting method. Incorporate a slow-release calcium source (e.g., CaCO₃) and a weak acid (e.g., GDL) into the alginate solution. Gelation occurs homogeneously upon dissolution and acidification.
- Chemical Crosslinking (e.g., Chitosan-Genipin):
- Lab Scale: Directly add Genipin solution to chitosan solution with stirring.
- Scale-up Challenge: Ensuring homogeneous mixing before gelation sets in.
- Scale-up Solution: Utilize a dynamic mixing head or static mixer for rapid and homogeneous introduction of the crosslinker into the polymer stream. Monitor the reaction by the development of a blue pigment.
- Enzymatic Crosslinking (e.g., Protein-based with MTGase):
- Lab Scale: Add enzyme to protein solution (e.g., gelatin) and incubate at 37°C.
- Scale-up Challenge: Maintaining consistent temperature and enzyme activity throughout a large batch.
- Scale-up Solution: Use a jacketed bioreactor for precise temperature control. Pre-determine enzyme kinetics to define mixing and holding times.
3.2.4. Data Collection and Analysis:
- Gelation Time: As in Protocol 3.1.4.
- Crosslinking Density: Estimate by measuring the effective crosslink density (νe) from compression data using the rubber elasticity theory: νe ≈ G' / (RT), where R is the gas constant and T is the absolute temperature.
- Swelling Ratio: As in Protocol 3.1.4. A lower swelling ratio typically indicates a higher crosslinking density.
Table 2: Comparison of Crosslinking Strategies for Scale-up
| Crosslinking Type | Mechanism | Scalability Rating | Key Scaling Challenge | Recommended Scale-up Solution |
|---|---|---|---|---|
| Ionic | Divalent cation diffusion (e.g., Ca²âº) | Medium | Homogeneous gelation in large volumes | Internal setting method with GDL/CaCO₃ [82] |
| Chemical (Covalent) | Covalent bond formation (e.g., Genipin) | High (with equipment) | Rapid, uncontrolled reaction leading to heterogeneity | In-line static mixing for instantaneous mixing [81] |
| Enzymatic | Catalytic formation of covalent bonds | Medium | Enzyme cost and activity maintenance | Use of jacketed bioreactors for temperature control [82] |
| Photo-Crosslinking | Radical polymerization initiated by light | Low | Limited light penetration in large batches | Suitable primarily for thin films/coatings [83] |
The Scientist's Toolkit: Research Reagent Solutions
Selecting the appropriate materials is foundational to reproducible hydrogel fabrication. The following table details key reagents and their functions.
Table 3: Essential Research Reagents for Reproducible Hydrogel Fabrication
| Reagent / Material | Function / Purpose | Key Consideration for Scaling |
|---|---|---|
| Natural Polymers (Alginate, Chitosan) [8] [83] | Base scaffold material; provides biocompatibility and biodegradability. | High batch-to-batch variability in natural sourcing requires stringent supplier qualification and in-house testing. |
| Synthetic Polymers (PEG, PVA) [84] [83] | Base scaffold material; offers tunable mechanical properties and high reproducibility. | Requires precise control over molecular weight distribution and end-group functionality during synthesis. |
| Ionic Crosslinkers (CaClâ‚‚, Fe³âº) [82] [83] | Forms hydrogel networks via ionic interactions; often reversible. | Purity is critical. Scaling the "drop-by-drop" method is infeasible; internal setting or aerosol spraying required. |
| Chemical Crosslinkers (Genipin, Glutaraldehyde) [83] | Forms permanent, covalent hydrogel networks; increases mechanical strength. | Genipin is less cytotoxic than glutaraldehyde. Homogeneous mixing at scale is the primary challenge. |
| Enzymatic Crosslinkers (Microbial Transglutaminase) [82] | Forms biocompatible covalent bonds under mild conditions. | Enzyme activity per batch must be calibrated. Cost can be prohibitive for very large-scale production. |
| Bioactive Cargo (Growth Factors, Exosomes) [33] [8] | Confers therapeutic functionality (e.g., promotes angiogenesis). | Sensitive to processing conditions (heat, shear). Loading homogeneity and release kinetics are difficult to control at scale. |
Visualization of Integrated Scaling Strategy
A successful scale-up strategy requires the integration of material selection, fabrication technology, and quality control.
Diagram 2: An integrated strategy for scalable hydrogel manufacturing.
Scaling hydrogel production for wound healing requires a systematic and integrated approach that moves beyond formulation to address the intricacies of manufacturing science. By implementing the standardized protocols and analytical methods outlined here—focusing on quantitative monitoring of batch consistency, optimized crosslinking strategies, and rigorous quality control—researchers and developers can significantly enhance process reproducibility. Overcoming these manufacturing hurdles is the critical final step in translating advanced hydrogel technologies from the laboratory bench to the patient's bedside, ensuring that these innovative biomaterials can reliably deliver on their promise to improve wound care outcomes.
Evidence and Efficacy: Preclinical and Clinical Validation of Hydrogel Therapies
In Vitro and In Vivo Models for Assessing Wound Healing Efficacy
The evaluation of wound healing efficacy is a critical component in the development of advanced therapeutic agents, particularly in the context of hydrogel fabrication for wound care. Researchers and drug development professionals require robust, standardized experimental models that can accurately predict clinical performance. This document provides detailed application notes and protocols for the key in vitro and in vivo models used to assess wound healing potential, with a specific focus on integrating these methodologies into hydrogel development workflows. The selection of appropriate models enables the systematic investigation of cellular migration, tissue regeneration, and the molecular mechanisms underlying repair processes, providing essential preclinical data for therapeutic advancement.
The wound healing assay, particularly the in vitro scratch assay, serves as a fundamental first-line assessment for evaluating cellular migration capabilities—a critical parameter for any pro-healing therapeutic. For more comprehensive evaluation, in vivo models provide the necessary biological complexity to assess tissue regeneration, angiogenesis, and immune response in a physiologically relevant environment. When developing hydrogel-based treatments, combining these approaches allows researchers to establish clear correlations between material properties and biological performance, accelerating the translation of innovative wound care solutions from bench to bedside.
Comparison of Wound Healing Assessment Models
Table 1: Characteristics of Primary Wound Healing Assessment Models
| Model Type | Key Applications | Advantages | Limitations | Throughput |
|---|---|---|---|---|
| In Vitro: Scratch Wound Assay | Measurement of cell migration; Screening therapeutic compounds; Studying genetic modifications on motility [85] | Simple, cost-effective; Provides quantitative data on cell motility; Real-time visualization; No specialized equipment required [85] | Lacks in vivo complexity; Manual creation can introduce variability; Cell proliferation may confound migration measurements [85] | Medium |
| In Vivo: Diabetic Rat Model | Evaluation of full-thickness wound closure; Assessment of tissue regeneration; Studying impaired healing pathologies [86] | Captures systemic factors; Clinically relevant for diabetic wound studies; Allows histopathological analysis [86] | Ethical considerations; Higher costs; Technical complexity; Longer experimental timelines [87] | Low |
| AI-Enhanced Image Analysis | Automated wound segmentation; Quantification of closure dynamics; Prediction of healing trajectories [88] [89] | Reduces subjectivity; Enables high-throughput analysis; Captures temporal patterns; Improves reproducibility [88] [89] | Requires technical expertise; Dependent on image quality; Model training needed [88] | High |
Quantitative Outcomes from Representative Studies
Table 2: Efficacy Metrics from Recent Wound Healing Studies
| Intervention | Model System | Key Efficacy Metrics | Outcome vs Control | Source |
|---|---|---|---|---|
| SDF-1α/LLC Hydrogel | In vitro (HDF cells) | Cell migration at 24h | 32.8% improvement (P < 0.001) [86] | |
| SDF-1α/LLC Hydrogel | In vivo (Diabetic rats) | Wound closure at day 14 | 51.4% faster closure (P < 0.001) [86] | |
| Immuno-regulating Hydrogels | Clinical (DFU patients) | Complete wound closure | Highest effect estimate (2.2, 95% CI: 1.6, 3.2) [90] | |
| Anti-bacterial Hydrogels | Clinical (DFU patients) | Complete wound closure | Lower effect estimate (1.3, 95% CI: 0.78, 2.3) [90] | |
| UNet++ Segmentation | In vitro (MCF10A & MCF7 cells) | Segmentation accuracy | Significantly higher than Otsu (Dice: p = 8.841 × 10â»â´â¹) [88] |
In Vitro Assessment: Scratch Wound Healing Assay
Principle and Applications
The scratch wound healing assay, also known as the in vitro scratch assay, is a widely used method to measure cell migration and wound closure in cultured cell monolayers [85]. This protocol involves creating a "wound" or gap in a confluent cell monolayer and monitoring the movement of cells into the cleared area over time. It is a simple, cost-effective method that provides quantitative data on cell motility, making it ideal for evaluating the effects of drugs, growth factors, or genetic modifications [85]. The assay enables analysis of whole cell masses for collective migration as well as individual cell migration, depending on the experimental setup, and is extensively used in cancer research to evaluate the metastatic potential of tumor cells and in regenerative medicine to assess healing potential [85].
Detailed Experimental Protocol
Materials Required:
- Confluent flask or plate of cells
- 12-well culture plates
- Appropriate growth medium
- Serum-free media
- Enzymatic or chemical cell detachment agent
- Standard cell culture consumables
- Treatments being assessed
- Light microscope with attached digital camera
- Thin tipped permanent marker
- 200 µL pipette tips [85]
Methodology:
Cell Culture Preparation:
- Using a confluent plate or flask of cells, aspirate the media and dispose.
- Add an appropriate volume of PBS, ensuring the cells are covered.
- Gently swirl the plate, then aspirate and discard PBS.
- Add an appropriate amount of serum-free media (10-12 mL) and culture the cells for 24 h [85].
Cell Collection and Seeding:
- Aspirate media and dispose.
- Wash with PBS, then add appropriate detachment reagent and incubate at 37°C until cells are detached.
- Add fresh culture media and transfer cell suspension to a centrifuge tube.
- Centrifuge at 200-250 x g for 5 min.
- Remove media and resuspend the cell pellet in an appropriate volume of PBS.
- Centrifuge again at 200-250 x g for 5 min.
- Aspirate and discard PBS, then resuspend cell pellet in fresh growth medium.
- Transfer the required volume of cells to each well of a 12-well plate such that the cells would be ~80% confluent after 24 h.
- Add serum-free media to the required volume (1-2 mL) and culture the cells for 24 h.
- At this point, add any treatments you are assessing, if they require a pre-treatment period [85].
Wound Creation:
- Using a 200 µL pipette tip, scratch the monolayer of confluent cells in the center and along the entirety of the well's diameter.
- Do not be too firm with the tip, as this can result in plastic residue being transferred from the tip to the plate.
- Ensure that the tip makes contact with the bottom of the plate along the entire length of the scratch [85].
Post-Scratch Processing:
- Aspirate the media from each well and dispose.
- Gently add an appropriate volume of PBS, ensuring the cells are covered.
- Gently swirl the plate, then aspirate and discard PBS. Ensure any detached cells and debris are removed.
- Add an appropriate amount of serum-free culture media (1-2 mL).
- At this point, add any treatments you are assessing—assuming they do not require a pre-treatment period [85].
Image Acquisition:
- Using a light microscope with an attached digital camera, focus on the wound area.
- Ensuring a suitable area of the monolayer of cells is visible on either side of the wound with the wound in the center, obtain an image of the cells.
- Using a thin-tipped permanent marker, carefully mark on the bottom or top of the culture plate where the image was taken.
- Repeat this for each well.
- Return cells to a culture incubator and culture for 24 h [85].
Time-Course Imaging:
- Using a light microscope with an attached digital camera, focus on the wound areas marked previously.
- Ensuring a suitable area of the monolayer of cells is visible on either side of the wound with the wound in the center, obtain an image of the cells.
- Repeat this for each well.
- If you are observing wound closure for longer than 24 h, replace media/treatments if required and return cells to a culture incubator and culture for your designated time period.
- Repeat the imaging steps as required [85].
Wound Closure Quantification:
- Ensure the digital images obtained are named appropriately and filed accordingly.
- Using a tool like ImageJ, measure the width of the wound in the images taken after scratching (a).
- Using the image from the same well after allowing for wound closure, measure the width of the wound following closure (b).
- The percentage of wound closure can be calculated as follows:
- % wound closure = (a-b)/a × 100 [85]
Advanced AI-Enhanced Analysis
Recent advancements have integrated artificial intelligence for more precise quantification of wound closure dynamics. Enhanced UNet++ models have demonstrated significantly higher segmentation accuracy than traditional Otsu thresholding (Dice: p = 8.841 × 10â»â´â¹; IoU: p = 3.931 × 10â»â´â·) with consistent temporal robustness across healing phases [88]. For closure trajectory modeling, Random Forest algorithms have achieved superior accuracy for both MCF7 (mean absolute error [MAE] = 0.48 %, R² = 0.968) and MCF10A (MAE = 1.73 %, R² = 0.872) cell lines, excelling in capturing nonlinear phase transitions and plateau behaviors [88].
AI-enhanced wound closure analysis workflow
In Vivo Assessment: Diabetic Wound Healing Model
Principle and Applications
In vivo models provide essential physiological context that cannot be captured in vitro, particularly for evaluating hydrogel-based therapies. The diabetic rat model with full-thickness wounds specifically addresses the complexity of impaired healing environments, mirroring key aspects of human diabetic foot ulcers [86]. This model allows researchers to assess not only wound closure rates but also tissue regeneration quality, angiogenesis, immune response, and the performance of therapeutic hydrogels in a pathologically relevant setting. Recent research highlights the critical importance of selecting appropriate in vivo models that implement the hallmarks of chronic wounds to yield translatable results [87].
Detailed Experimental Protocol
Materials Required:
- Diabetic rats (streptozotocin-induced or genetic models)
- Anesthetic agents (ketamine/xylazine)
- Clippers and hair removal cream
- Biopsy punch (6-8 mm)
- Test articles (hydrogel formulations)
- Control dressings (e.g., PHMB)
- Digital camera system
- Histopathology supplies (fixatives, processing reagents, staining solutions) [86]
Methodology:
Animal Preparation:
- Induce diabetes in rats using streptozotocin (typically 55-65 mg/kg intraperitoneally) and confirm hyperglycemia (blood glucose >250 mg/dL) after 72 hours.
- Anesthetize animals using appropriate anesthetic protocol (e.g., ketamine 80 mg/kg and xylazine 10 mg/kg intraperitoneally).
- Remove hair from the dorsal area using clippers followed by depilatory cream.
- Clean the surgical area with alternating betadine and alcohol scrubs (three cycles). [86]
Wound Creation:
- Create full-thickness excisional wounds using a sterile biopsy punch (6-8 mm diameter).
- Extend the wound through the panniculus carnosus muscle layer to ensure full-thickness injury.
- Apply gentle pressure with gauze to achieve hemostasis.
- Assign animals randomly to treatment groups (typically n=10 per group). [86]
Treatment Application:
- Apply test articles directly to wound beds:
- Experimental hydrogel formulations (e.g., SDF-1α/LLC hydrogel)
- Control hydrogel (LLC hydrogel without active factor)
- Standard care control (e.g., PHMB)
- Negative control (no treatment or vehicle)
- Cover with appropriate secondary dressing.
- Replace dressings and reapply treatments according to experimental schedule (typically every 2-3 days). [86]
- Apply test articles directly to wound beds:
Wound Monitoring and Assessment:
- Monitor animals daily for signs of infection or distress.
- Capture wound images on days 0, 3, 7, 14, and 21 using standardized photography setup (fixed distance, lighting, and reference scale).
- Calculate wound area using image analysis software (e.g., ImageJ):
- % Wound closure = [(Initial area - Time point area)/Initial area] × 100
- Document clinical observations of granulation tissue formation, epithelialization, and exudate. [86]
Tissue Collection and Histological Analysis:
- Euthanize animals at predetermined endpoints using approved method (e.g., COâ‚‚ asphyxiation followed by cervical dislocation).
- Harvest wound tissue with 2-3 mm margin of surrounding unwounded skin.
- Bisect specimens: one half for histological processing, one half for molecular analysis.
- Fix tissue in 10% neutral buffered formalin for 24-48 hours.
- Process through graded ethanol series, embed in paraffin, and section at 5 µm thickness.
- Perform staining with:
- Hematoxylin and Eosin (H&E) for general morphology
- Masson's Trichrome for collagen deposition assessment
- Immunohistochemistry for specific markers (e.g., CD31 for angiogenesis, CD68 for macrophages) [86]
Histopathological Scoring:
- Evaluate sections using semi-quantitative scoring systems for:
- Epithelialization (0-4 scale)
- Granulation tissue formation (0-4 scale)
- Collagen organization (0-4 scale)
- Inflammatory cell infiltration (0-4 scale)
- Neovascularization (0-4 scale)
- Perform blinded evaluation by multiple independent observers. [86]
- Evaluate sections using semi-quantitative scoring systems for:
The Scientist's Toolkit: Essential Research Reagents and Materials
Table 3: Key Research Reagent Solutions for Wound Healing Studies
| Reagent/Material | Function | Application Notes | Representative Examples |
|---|---|---|---|
| Lipid Liquid Crystalline (LLC) Hydrogel | Drug delivery matrix; Provides sustained release of bioactive factors [86] | Protects encapsulated agents from degradation; Enables controlled release; Enhances tissue regeneration [86] | SDF-1α/LLC hydrogel demonstrated 51.4% faster wound closure vs control in diabetic rats [86] |
| Immuno-regulating Hydrogels (IRH) | Modulates wound immune microenvironment; Promotes transition from inflammatory to proliferative phase [90] | Addresses chronic inflammation in diabetic wounds; Highest efficacy ranking in network meta-analysis [90] | Effect estimate: 2.2 (95% CI: 1.6, 3.2) for complete wound closure in DFU patients [90] |
| Stromal Cell-Derived Factor-1α (SDF-1α) | Chemotactic factor; Recruits progenitor cells; Promotes angiogenesis [86] | Enhances fibroblast migration and proliferation; Critical for stem cell homing to wound sites [86] | SDF-1α/LLC hydrogel significantly enhanced HDF cell migration (32.8% improvement over control) [86] |
| UNet++ Architecture | Semantic segmentation of wound areas; Precise boundary detection in time-lapse images [88] | Superior to traditional thresholding methods; Enables automated, high-throughput analysis [88] | Significantly outperformed Otsu thresholding (Dice: p = 8.841 × 10â»â´â¹) [88] |
| Random Forest Algorithm | Temporal modeling of wound closure dynamics; Prediction of healing trajectories [88] | Captures nonlinear phase transitions; Handles plateau behaviors in closure kinetics [88] | Superior accuracy for MCF7 (MAE = 0.48 %, R² = 0.968) and MCF10A (MAE = 1.73 %, R² = 0.872) [88] |
Integrated Assessment Strategy
The most comprehensive evaluation of hydrogel efficacy comes from integrating multiple assessment approaches. The following diagram illustrates the strategic workflow for connecting in vitro and in vivo models:
Integrated wound healing assessment strategy
This integrated approach enables researchers to establish correlations between in vitro performance and in vivo efficacy, accelerating the development of effective hydrogel-based therapies. The implementation of standardized reporting guidelines, such as the Wound Reporting in Animal and Human Preclinical Studies (WRAHPS) Guidelines, ensures consistency, transparency, and reproducibility across studies, facilitating comparative analysis and meta-analyses [91].
By employing these standardized protocols and assessment strategies, researchers can systematically evaluate the wound healing efficacy of novel hydrogel formulations, generating robust, reproducible data to support therapeutic development and regulatory approval.
1.0 Clinical Outcomes Data Summary Quantitative findings from a systematic review and meta-analysis of 43 clinical studies (n=3,521 patients) are summarized below, comparing hydrogel dressings to non-hydrogel controls [92] [93].
Table 1: Summary of Clinical Effectiveness of Hydrogel Dressings
| Wound Type | Outcome Measure | Result (Hydrogel vs. Control) | Statistical Significance (p-value) | Number of Studies (Patients) |
|---|---|---|---|---|
| Degree II Burns | Healing Time | Significantly Shortened | p < 0.05 | Multiple RCTs/CCTs |
| Pain Relief | Significantly Improved | p < 0.05 | Multiple RCTs/CCTs | |
| Diabetic Foot Ulcers | Healing Time | Significantly Shortened | p < 0.05 | Multiple RCTs/CCTs |
| Cure Rate | Significantly Increased | p < 0.05 | Multiple RCTs/CCTs | |
| Traumatic Skin Injuries | Healing Time | Significantly Shortened | p < 0.05 | Multiple RCTs/CCTs |
| Pain Relief | Significantly Improved | p < 0.05 | Multiple RCTs/CCTs | |
| Surgical Wounds | Cure Rate | Significantly Increased | p < 0.05 | Multiple RCTs/CCTs |
| Radioactive Skin Injuries | Healing Time | Significantly Shortened | p < 0.05 | Multiple RCTs/CCTs |
| Inpatients' Pressure Ulcers | Cure Rate | No Significant Difference | p > 0.05 | Multiple RCTs/CCTs |
2.0 Experimental Protocol: In Vitro Hydrogel Swelling and Drug Release
This protocol details the methodology for evaluating fundamental properties of fabricated hydrogels relevant to their function as drug-eluting wound dressings.
2.1 Reagents and Materials
- Hydrogel Formulation: Polymer (e.g., Carboxy Methyl Cellulose, Guar Gum), cross-linker, propylene glycol, purified water [92] [93].
- Test Solution: Phosphate Buffered Saline (PBS), pH 7.4, to simulate wound exudate.
- Active Agent: Model drug or growth factor (e.g., recombinant growth factors, antibacterial agents) [18].
2.2 Procedure
- Hydrogel Fabrication: Synthesize hydrogel sheets (e.g., via cross-linking 2-hydroxyethyl methacrylate or natural polymers) with a standardized thickness of 2 mm [92].
- Swelling Ratio:
- Weigh initial mass of dry hydrogel disc (Wd).
- Immerse the disc in PBS at 37°C.
- At predetermined time intervals, remove the hydrogel, blot gently to remove surface liquid, and record the swollen mass (Ws).
- Calculate the swelling ratio as (Ws - Wd) / Wd. Perform in triplicate.
- Drug Release Kinetics:
- Load the hydrogel with a known concentration of the active agent during fabrication.
- Immerse the loaded hydrogel in a known volume of PBS at 37°C under gentle agitation.
- At scheduled time points, withdraw aliquots of the release medium and analyze the concentration of the active agent using a calibrated method (e.g., UV-Vis spectroscopy, HPLC).
- Replenish with fresh PBS to maintain sink conditions. Perform in triplicate.
2.3 Data Analysis
- Plot swelling ratio versus time to determine equilibrium swelling capacity.
- Plot cumulative drug release (%) versus time to establish release profile (e.g., burst release, sustained release).
In-vitro Hydrogel Characterization Workflow
3.0 Mechanism of Action: Hydrogel Function in Wound Healing Hydrogels promote healing through multiple interconnected mechanisms within the wound microenvironment [92] [18].
Hydrogel Mechanisms in Wound Healing
4.0 The Scientist's Toolkit: Research Reagent Solutions
Table 2: Essential Materials for Hydrogel Wound Dressing Research
| Item | Function/Description | Research Application |
|---|---|---|
| Natural Polymers (e.g., Alginate, Chitosan, CMC) | Base material providing hydrophilicity, biocompatibility, and biodegradability. Forms the 3D network [92] [18]. | Fabrication of the hydrogel matrix. |
| Synthetic Polymers (e.g., PVP, PVA, PEG) | Offer tunable mechanical properties, controlled degradation rates, and high modifiability [18]. | Creating hybrid or fully synthetic hydrogels with enhanced strength. |
| Cross-linkers (e.g., Glutaraldehyde, Genipin, UV-initiators) | Agents that create covalent or ionic bonds between polymer chains, stabilizing the hydrogel structure. | Controlling gelation time, mechanical strength, and swelling behavior. |
| Propylene Glycol | Acts as a humectant and possesses bacteriostatic properties, helping to prevent bacterial proliferation [92] [93]. | Formulating the hydrogel's liquid component to maintain a moist environment. |
| Bioactive Agents (e.g., Growth Factors, Antibiotics) | Therapeutic compounds incorporated to actively promote healing (angiogenesis, cell proliferation) or prevent infection [92] [18]. | Creating advanced, functionalized dressings for specific wound types (e.g., chronic, infected). |
| Cell Culture Assays (Fibroblasts, Keratinocytes) | In vitro models to assess hydrogel cytotoxicity and its ability to support cell migration and proliferation. | Evaluating biocompatibility and bioactivity before animal or clinical studies. |
Within the broader context of research on hydrogel fabrication for wound healing, selecting an appropriate wound dressing is a critical determinant of clinical outcomes. The ideal dressing must manage exudate, prevent infection, and actively promote the coordinated cellular events of the healing cascade [18]. Traditional dressings, primarily gauze and bandages, have long been the standard of care but often fall short of creating an optimal healing environment [94]. The development of advanced dressings, including films, foams, hydrocolloids, and hydrogels, represents a significant evolution in wound management. These materials are engineered to interact with the wound bed, maintaining moisture and facilitating autolytic debridement [95]. Among these, hydrogels have garnered significant research interest due to their unique three-dimensional hydrophilic polymer networks, which can absorb and retain large volumes of water while maintaining structural integrity [82]. This application note provides a comparative analysis of these dressing categories, with a specific focus on the properties, mechanisms, and applications of hydrogels, supported by quantitative data and detailed experimental protocols relevant to researchers and drug development professionals.
Wound Healing Physiology and Dressing Requirements
A thorough understanding of wound healing physiology is fundamental to the rational design and selection of wound dressings. The process is a complex, overlapping sequence of four phases: hemostasis, inflammation, proliferation, and remodeling [18] [8].
- Hemostasis: Immediately following injury, vasoconstriction occurs and a fibrin-based clot forms, providing a provisional matrix for cell migration [18] [33].
- Inflammation: Characterized by the infiltration of neutrophils and macrophages to clear pathogens and debris. This phase is typically prolonged in chronic wounds [18] [94].
- Proliferation: Fibroblasts synthesize new extracellular matrix (ECM), angiogenesis occurs, and epithelial cells migrate to cover the wound [8].
- Remodeling: Collagen is realigned and cross-linked to increase tensile strength, a process that can continue for over a year [18].
Chronic wounds, such as diabetic foot ulcers, venous leg ulcers, and pressure ulcers, are defined by a failure to progress through these stages in a timely and orderly manner, often stalling in the inflammatory phase [94]. Key pathophysiological features include persistent inflammation, elevated levels of matrix metalloproteinases (MMPs), bacterial bioburden with biofilm formation, and impaired angiogenesis [95] [1]. An ideal dressing must therefore address these challenges by providing a moist environment, managing exudate without desiccating the wound, allowing gas exchange, protecting from infection, and being non-adherent to avoid trauma upon removal [72].
Comparative Analysis of Wound Dressings
The following table provides a systematic comparison of the key characteristics of traditional dressings, modern advanced dressings, and hydrogel-based dressings.
Table 1: Comparative Analysis of Wound Dressing Types
| Dressing Type | Key Characteristics | Mechanism of Action | Advantages | Disadvantages | Ideal Wound Types |
|---|---|---|---|---|---|
| Traditional (Gauze, Bandages) [94] [72] | Passive, dry dressings made of woven or non-woven fibers. | Absorption of exudate into the fibers. | Low cost, readily available, highly absorbent for heavily exudating wounds. | Can adhere to the wound bed, causing trauma and pain on removal; require frequent changes; can lead to wound desiccation. | Dry wounds requiring debridement; as secondary dressings. |
| Semi-permeable Films [94] | Thin, transparent, adhesive polyurethane sheets. | Provide a barrier to bacteria and external contaminants while allowing moisture vapor and oxygen transmission. | Transparent, allowing for wound monitoring without removal; waterproof. | Non-absorbent; can lead to maceration of surrounding skin; not suitable for uneven surfaces. | Superficial wounds with low exudate, such as partial-thickness burns or donor sites. |
| Hydrocolloids [94] | Dressings composed of gelatin, pectin, and carboxymethylcellulose. | Interaction with wound exudate forms a gel, creating a moist environment. | Provide autolytic debridement; waterproof and bacteria-proof; can be left in place for several days. | Opaque, preventing wound inspection; may cause an odor that can be mistaken for infection; not for heavily exudating wounds. | Light- to moderately-exudating wounds, such as pressure ulcers and venous leg ulcers. |
| Foams [94] | Polyurethane or silicone sheets with absorbent properties. | Exudate is absorbed into the foam's air bubbles. | Highly absorbent; provide thermal insulation; non-adherent and soft. | Opaque; may require a secondary dressing; can be too absorbent for low-exudate wounds, leading to desiccation. | Moderate to heavily exudating wounds, such as diabetic foot ulcers and venous leg ulcers. |
| Hydrogels (Standard) [18] [94] [36] | 3D networks of hydrophilic polymers (e.g., alginate, chitosan, PEG) with high water content (>90%). | Donate moisture to the wound bed, creating a moist environment that facilitates autolytic debridement and cell migration. | Soothing, cooling effect; non-adherent; promote autolytic debridement; biocompatible. | Low mechanical strength; limited fluid-handling capacity; may require a secondary dressing. | Dry to minimally exudating wounds, necrotic wounds, and burns. |
| Advanced Bioactive Hydrogels [33] [36] [1] | Hydrogels functionalized with bioactive agents (e.g., antimicrobials, growth factors). | Combines the physical properties of standard hydrogels with active therapeutic release and immunomodulation. | Can be engineered for controlled drug delivery; modulate the wound microenvironment (e.g., scavenge ROS); promote angiogenesis. | Complex fabrication; higher cost; stability and release kinetics of bioactive agents must be carefully controlled. | Complex chronic wounds (e.g., infected diabetic foot ulcers, refractory venous leg ulcers). |
Quantitative data from clinical studies further underscores the efficacy of hydrogels. A systematic review of 39 clinical trials concluded that hydrogel dressings enhanced the percentage of wound closure by 63.76 ± 28.97% and reduced the required healing time by 31.17 ± 21.74 days compared to non-hydrogel dressings [96].
Detailed Experimental Protocols for Hydrogel Evaluation
For researchers developing new hydrogel-based dressings, the following protocols outline key in vitro and in vivo experiments to characterize material properties and biological efficacy.
Protocol 1:In VitroSwelling and Degradation Kinetics
Objective: To quantify the water absorption capacity and enzymatic degradation profile of fabricated hydrogels.
Materials:
- Research Reagent Solutions:
- Phosphate Buffered Saline (PBS), pH 7.4: Simulates physiological ionic conditions for swelling studies.
- Lysozyme or Collagenase Solutions: Enzymes to simulate the proteolytic environment of a chronic wound for degradation studies.
- Hydrogel specimens (e.g., disks of 10 mm diameter).
- Analytical balance (precision ±0.01 mg).
- Incubator maintained at 37°C.
Procedure:
- Initial Weight Measurement: Pre-weigh dry hydrogel disks (Wd).
- Swelling Study: Immerse specimens in PBS at 37°C. At predetermined time intervals, remove samples, gently blot with filter paper to remove surface liquid, and record the wet weight (Ws). Calculate the swelling ratio (SR) using: SR (%) = [(Ws - Wd) / Wd] × 100.
- Degradation Study: After equilibrium swelling, transfer samples to vials containing PBS with a specific concentration of lysozyme (e.g., 1.5 µg/mL) or collagenase (e.g., 100 U/mL). Maintain at 37°C under agitation.
- Mass Loss Measurement: At regular intervals, remove samples, rinse with distilled water, dry completely, and weigh (Wt). Calculate the remaining mass (RM) using: RM (%) = (Wt / Wd) × 100.
- Data Analysis: Plot SR and RM over time to determine kinetics. Fit degradation data to mathematical models (e.g., first-order) to predict long-term behavior.
Protocol 2:In VivoEvaluation in a Diabetic Murine Wound Model
Objective: To assess the efficacy of a bioactive hydrogel in promoting healing in a compromised wound model.
Materials:
- Research Reagent Solutions:
- Streptozotocin (STZ): Agent for inducing type 1 diabetes.
- Isoflurane: Inhalant anesthetic for humane animal procedures.
- Test Article: Bioactive hydrogel (e.g., chitosan hydrogel loaded with silver nanoparticles).
- Control Articles: Standard hydrogel (no bioactivity), traditional gauze dressing.
- C57BL/6 mice (8-10 weeks old).
- Digital calipers and high-resolution camera.
Procedure:
- Diabetes Induction: Inject mice intraperitoneally with a citrate-buffered STZ solution (e.g., 50 mg/kg for 5 consecutive days). Confirm hyperglycemia (blood glucose >250 mg/dL) one week post-injection.
- Wound Creation: Anesthetize diabetic mice and create full-thickness excisional wounds (e.g., 8 mm diameter) on the dorsum.
- Treatment Application: Randomize animals into groups (n≥6) and apply the test article, standard hydrogel, or gauze control to the wounds. Secure with a semi-occlusive film dressing.
- Wound Monitoring: Photograph wounds daily with a scale reference. Measure wound area using image analysis software (e.g., ImageJ). Calculate percent wound closure: % Closure = [(A0 - At) / A0] × 100, where A0 is initial area and At is area at time t.
- Histological Analysis: At endpoints (e.g., days 7 and 14), euthanize animals and harvest wound tissue. Process for H&E staining (to assess re-epithelialization and granulation tissue) and Masson's Trichrome staining (to assess collagen deposition and maturation).
- Data Analysis: Compare wound closure rates and histological scores between groups using appropriate statistical tests (e.g., ANOVA with post-hoc analysis).
Visualizing the Research Workflow and Dressing Selection
The following diagrams illustrate the logical workflow for hydrogel development and the decision-making process for clinical dressing selection.
The Scientist's Toolkit: Essential Research Reagents and Materials
Table 2: Key Research Reagents for Hydrogel Fabrication and Testing
| Research Reagent / Material | Function & Application | Example Sources / Notes |
|---|---|---|
| Natural Polymers (Chitosan, Alginate, Collagen) | Base materials for hydrogel fabrication; provide biocompatibility, biodegradability, and inherent bioactivity. | Sigma-Aldrich, Merck; sourced from crustacean shells (chitosan), brown algae (alginate), or bovine/porcine tissue (collagen). |
| Synthetic Polymers (Polyethylene Glycol (PEG), Polyvinyl Alcohol (PVA)) | Offer tunable mechanical properties and controlled chemical structure; often used in hybrid hydrogels. | Sigma-Aldrich, Merck; available in various molecular weights, influencing hydrogel crosslinking density and swelling. |
| Crosslinking Agents (Genipin, Glutaraldehyde, CaClâ‚‚) | Form stable 3D networks by creating covalent or ionic bonds between polymer chains. | Sigma-Aldrich, Merck; genipin is a less cytotoxic alternative to glutaraldehyde; CaClâ‚‚ is used for ionotropic gelation of alginate. |
| Bioactive Agents (Silver Nanoparticles, Growth Factors, Antibiotics) | Impart specific therapeutic functions (antimicrobial, pro-angiogenic) to the hydrogel matrix. | Nanocomposix (nanoparticles); PeproTech (growth factors like VEGF, FGF); antibiotics should be selected based on target pathogens. |
| Matrix Metalloproteinase (MMP) Substrates | Used in in vitro assays to quantify and characterize the degradability of hydrogels in a simulated chronic wound environment. | R&D Systems; fluorescently-tagged collagen or gelatin can be incorporated into hydrogels to monitor enzyme activity. |
This comparative analysis underscores that hydrogel-based dressings occupy a unique and versatile position in the wound care landscape. Their fundamental ability to maintain a moist wound environment, facilitate autolytic debridement, and promote cellular migration provides a superior healing platform compared to traditional passive dressings for many wound types [18] [96] [94]. The emergence of advanced bioactive hydrogels, which can be engineered to deliver drugs, scavenge reactive oxygen species, and modulate the immune response, represents the cutting edge of wound management, particularly for complex chronic wounds [33] [1]. The experimental protocols and research tools detailed herein provide a framework for the continued development and rigorous evaluation of next-generation hydrogel dressings. Future research directions will likely focus on optimizing fabrication technologies like 3D bioprinting for personalized dressings, enhancing the controlled release kinetics of multiple bioactive agents, and improving the mechanical properties of natural polymer-based hydrogels to expand their clinical applicability [82] [36]. The translation of these innovative hydrogel technologies from the laboratory to the clinic holds significant promise for improving outcomes for patients with acute and chronic wounds.
Clinical Performance: Quantitative Outcomes
This section synthesizes key quantitative findings from clinical trials evaluating hydrogel dressings against standard care and other modern dressings for chronic wounds, including diabetic foot ulcers (DFUs), pressure ulcers, venous leg ulcers, and burns.
Table 1: Healing Rates and Wound Size Reduction in Clinical Trials [4]
| Wound Type | Comparison (Hydrogel vs. Control) | Complete Healing Rate (%) | Wound Size Reduction / Time to Healing | Key Clinical Outcomes |
|---|---|---|---|---|
| Diabetic Foot Ulcers (DFUs) | Hydrogel vs. Basic Wound Care | 45.8% vs. 31.3% | Significant reduction in wound size | Effective for wounds with low exudate; promotes autolytic debridement [4] |
| Pressure Ulcers | Hydrogel vs. Saline Gauze | 41% vs. 5% | --- | Superior in wound bed preparation and healing progression [4] |
| Burn Wounds | Microbial Cellulose Hydrogel vs. Silver Sulphadiazine | --- | Reduced healing time | Enhanced re-epithelialization, lower pain scores [4] |
| Venous Leg Ulcers | Hydrogel vs. Standard Care | --- | --- | Improved healing trajectory; effective moisture management [4] |
| Surgical Wounds | Polyethylene Glycol Hydrogel as Dural Sealant | --- | --- | Demonstrated safety and efficacy in cranial surgery for complication prevention [4] |
Table 2: Patient-Centered Outcomes and Complication Management [4]
| Outcome Metric | Findings in Hydrogel Groups | Context and Implications |
|---|---|---|
| Pain Reduction | Significant reduction in pain scores during dressing changes | Hydrogels provide cooling sensation and are non-adherent, minimizing trauma and discomfort [4] |
| Pain Management | Effective alternative to topical anesthetics in burn care | Provides analgesia while supporting the healing environment [4] |
| Exudate Management | Effective for low to moderately exuding wounds | High water content hydrates dry wounds; composition can be tuned to manage moisture [4] [72] |
| Infection Control | Lower incidence of infection compared to standard care | Acts as a physical barrier; can be loaded with antimicrobial agents (e.g., silver) to disrupt bacterial membranes [4] [18] |
| Complication Profile | Low rates of adverse events (e.g., maceration, dermatitis) | Biocompatibility and non-immunogenic nature enhance safety profile [4] |
Experimental Protocols for Clinical Evaluation
Protocol: Randomized Controlled Trial for Hydrogel Dressing Efficacy
Objective: To compare the efficacy and safety of a novel hydrogel dressing against a standard-of-care dressing in patients with chronic diabetic foot ulcers.
Materials:
- Test Article: Investigational hydrogel dressing.
- Control Article: Standard foam or hydrocolloid dressing.
- Subjects: Adult patients with Type 1 or 2 diabetes and a Wagner Grade 1 or 2 DFU.
- Randomization: Computer-generated block randomization schedule.
Methodology:
- Screening & Consent: Obtain informed consent. Assess patient eligibility based on inclusion/exclusion criteria.
- Baseline Assessment: Document wound characteristics via high-resolution photography and planimetry. Record wound area, depth, and baseline pain score using a Visual Analog Scale (VAS).
- Randomization & Blinding: Randomize eligible patients to test or control group. Employ a single-blind design where the outcome assessor is blinded to the treatment assignment.
- Intervention: Apply the assigned dressing according to manufacturer's instructions. Standardize off-loading for all patients.
- Dressing Change & Follow-up: Perform dressing changes as per protocol (e.g., every 2-3 days or as indicated). At each visit:
- Assess wound for closure.
- Measure wound dimensions.
- Score pain during dressing removal and between changes.
- Document any adverse events (e.g., infection, maceration).
- Endpoint Assessment: The primary endpoint is the incidence of complete wound closure within 12 weeks. Secondary endpoints include percent reduction in wound area, time to complete healing, and changes in pain scores.
- Statistical Analysis: Perform intention-to-treat analysis. Use Chi-square test for complete healing rates and ANOVA/Repeated Measures ANOVA for continuous variables like wound area and pain scores.
Protocol: In Vitro Assessment of Antimicrobial Hydrogel Efficacy
Objective: To evaluate the antimicrobial activity of a drug-loaded hydrogel against common wound pathogens.
Materials:
- Hydrogel samples (unloaded and antimicrobial-loaded).
- Bacterial strains: Staphylococcus aureus (ATCC 25923), Pseudomonas aeruginosa (ATCC 27853).
- Culture media: Mueller-Hinton Agar (MHA) and Broth (MHB).
- Sterile phosphate-buffered saline (PBS).
Methodology:
- Sample Preparation: Aseptically prepare hydrogel discs of uniform size and weight.
- Inoculum Preparation: Adjust bacterial suspensions in PBS to a turbidity of 0.5 McFarland standard (~1.5 x 10^8 CFU/mL).
- Agar Disk Diffusion Test:
- Swab the surface of MHA plates with the standardized inoculum.
- Place hydrogel discs and a positive control antibiotic disk onto the inoculated agar.
- Incubate plates at 37°C for 18-24 hours.
- Measure the diameter of the zone of inhibition (ZOI) around each disc.
- Time-Kill Assay:
- Add hydrogel samples to flasks containing MHB inoculated with the test organism (~10^5 CFU/mL).
- Incubate at 37°C with shaking.
- Withdraw aliquots at predetermined time intervals (0, 2, 4, 6, 8, 24 hours), serially dilute, and plate on MHA.
- Count colony-forming units (CFU) after incubation to determine the reduction in viable bacteria over time.
- Analysis: Compare ZOI and bacterial reduction kinetics between unloaded and antimicrobial-loaded hydrogels.
Visualization of Hydrogel Mechanisms in Wound Healing
Diagram 1: Multimodal mechanism of hydrogel dressings in wound healing.
Diagram 2: Hydrogel-mediated shift from chronic to acute healing trajectory.
The Scientist's Toolkit: Research Reagent Solutions
Table 3: Essential Materials for Hydrogel Wound Dressing Research [4] [18] [79]
| Category / Reagent | Function in R&D | Examples & Rationale |
|---|---|---|
| Polymer Backbone | Forms the primary 3D network of the hydrogel. | Cellulose: Predominant in clinical trials; biocompatible and cost-effective [4].Chitosan: Inherent antimicrobial and hemostatic properties [8].Hyaluronic Acid: Key ECM component; promotes cell migration and proliferation [18].Poly(vinyl alcohol) (PVA): Synthetic polymer for tunable mechanical strength [79]. |
| Crosslinking Agents | Stabilizes the polymer network; defines mechanical properties and degradation. | Physical (e.g., freeze-thawing): Creates reversible, non-toxic networks [79].Chemical (e.g., genipin, glutaraldehyde): Forms permanent, strong covalent bonds [79]. |
| Bioactive Cargos | Confers specific therapeutic functions to the dressing. | Silver Nanoparticles (AgNPs): Broad-spectrum antimicrobial agent [8] [72].Growth Factors (e.g., VEGF, EGF): Directly promotes angiogenesis and re-epithelialization [18].Anti-inflammatory Drugs (e.g., dexamethasone): Counteracts chronic inflammation [18]. |
| Characterization Tools | Evaluates physical, chemical, and biological performance. | Rheometry: Quantifies viscoelasticity and mechanical modulus [97].Scanning Electron Microscopy (SEM): Visualizes internal porosity and microstructure [72].FTIR Spectroscopy: Confirms chemical structure and crosslinking [79].Live/Dead Assay & MTT Test: Assesses in vitro cytocompatibility [97]. |
Commercial Landscape and Regulatory Pathway for Hydrogel-Based Dressings
The global market for hydrogel-based wound dressings is experiencing robust growth, driven by an increasing prevalence of chronic wounds and advancements in material science [98] [99]. These dressings are prized for their ability to maintain a moist wound environment, facilitate autolytic debridement, and enhance patient comfort [18] [100].
Global Market Size and Growth Projections
Table 1: Hydrogel Dressing Market Size and Growth Forecasts from Various Sources
| Source | Base Year Market Size (USD) | Projected Year | Projected Market Size (USD) | CAGR |
|---|---|---|---|---|
| Data Insights Market [98] | 2025: 2,500 M | 2033 | 4,500 M | 7.5% (2025-2033) |
| S&S Insider [99] | 2024: 875.52 M | 2032 | 1,290.95 M | 4.99% (2025-2032) |
| Fortune Business Insights [100] | 2024: 791.3 M | 2032 | 1,209.7 M | 5.5% (2025-2032) |
| 360iResearch [101] [102] | 2024: 1.67 B | 2032 | 5.93 B | 17.12% (2025-2032) |
Note on Data Variance: The significant differences in market size values, particularly from 360iResearch, are likely due to varying segmentation definitions (e.g., some reports may include a broader range of "advanced wound care" products under the hydrogel dressing category) [101] [102].
Market Segmentation and Dominant Segments
Table 2: Key Market Segments and Leading Categories
| Segmentation Factor | Dominant Segment | Key Reasons for Dominance |
|---|---|---|
| Product Type [99] [100] | Amorphous Hydrogels | Versatility, ease of application to irregular wound beds, superior wound healing efficiency for dry, necrotic wounds [99] [100]. |
| Application [99] [100] | Chronic Wounds (Diabetic Foot Ulcers, Venous Leg Ulcers, Pressure Ulcers) | High and growing prevalence linked to aging and diabetes; require advanced care over long durations [98] [99] [100]. |
| End User [98] [99] [100] | Hospitals & Clinics | High flow of patients with complex wounds; availability of specialized wound care teams and reimbursement structures [99] [100]. |
| Region [98] [99] [100] | North America | Advanced healthcare infrastructure, high healthcare expenditure, strong reimbursement policies, and high prevalence of chronic conditions [99] [100]. |
Key Market Players and Innovation Trends
The competitive landscape is characterized by the presence of established medical device companies and ongoing innovation. Leading players include Smith & Nephew, 3M, ConvaTec Group PLC, Coloplast A/S, and Mölnlycke Health Care AB [99] [101] [102]. Key trends shaping the market include:
- Bioactive Integration: Development of hydrogels incorporating antimicrobial agents (e.g., silver), growth factors, and stem cell-targeted compounds to actively stimulate healing and prevent infection [18] [101] [33].
- Smart Dressings: Integration of sensor technologies for real-time monitoring of wound parameters like pH, temperature, and moisture levels [101] [102].
- Injectable and Sprayable Formulations: Advancements in in situ forming hydrogels that can conform to deep and irregular wounds, enabling minimally invasive application [101] [33].
- Material Sourcing and Tariffs: The market is adapting to U.S. tariff changes on medical device imports, leading to a shift towards regional manufacturing and resilient supply chains [101] [102].
Experimental Protocols: Preclinical Evaluation of Hydrogel Dressings
Protocol: In Vitro Biocompatibility and Bioactivity Assessment
Objective: To evaluate the cytotoxicity, cellular response, and drug release profile of a novel hydrogel dressing formulation.
Materials:
- Test Material: Sterile hydrogel dressing samples (e.g., 1 cm diameter discs).
- Cell Line: Human dermal fibroblasts (HDFs) or keratinocytes.
- Culture Media: Dulbecco's Modified Eagle Medium (DMEM) supplemented with 10% Fetal Bovine Serum (FBS) and 1% penicillin/streptomycin.
- Reagents: AlamarBlue or MTT assay kit, Live/Dead viability/cytotoxicity kit, ELISA kits for relevant growth factors (e.g., VEGF, FGF).
- Equipment: COâ‚‚ incubator, sterile tissue culture hood, microplate reader, fluorescence microscope.
Methodology:
- Extract Preparation: Incubate sterile hydrogel samples in complete cell culture media at a standard surface-area-to-volume ratio (e.g., 3 cm²/mL) for 24±2 hours at 37°C [18].
- Cell Seeding and Exposure: Seed HDFs in 96-well plates at a density of 1x10â´ cells/well and allow to adhere for 24 hours. Replace the media with the hydrogel extract. Use fresh culture media as a negative control and media with a cytotoxic substance (e.g., 1% Triton X-100) as a positive control.
- Cytotoxicity Assay (MTT/AlamarBlue):
- After 24-72 hours of exposure, add MTT or AlamarBlue reagent to the wells according to the manufacturer's instructions.
- Incubate for a predetermined time (e.g., 4 hours for MTT).
- Measure absorbance (MTT) or fluorescence (AlamarBlue) using a microplate reader. Cell viability is calculated as a percentage of the negative control.
- Live/Dead Staining: Following exposure, incubate cells with the Live/Dead stain (Calcein-AM for live cells, Ethidium homodimer-1 for dead cells) and visualize under a fluorescence microscope to assess cell membrane integrity and spatial distribution.
- Bioactivity/Drug Release Analysis (If applicable):
- For drug-loaded hydrogels, collect release media at predetermined time points and analyze drug concentration using HPLC or a relevant ELISA.
- Assess the biological effect of the released drug by measuring specific markers (e.g., collagen production via hydroxyproline assay, or growth factor expression via ELISA) in the exposed cells.
Protocol: In Vivo Efficacy in a Diabetic Wound Model
Objective: To assess the wound healing efficacy of a hydrogel dressing in a validated animal model of impaired healing.
Materials:
- Animals: Genetically diabetic (e.g., db/db) mice or rats, 8-12 weeks old.
- Test Article: Sterile hydrogel dressing, cut to wound size.
- Controls: Standard-of-care hydrogel dressing (positive control), saline-soaked gauze (negative control).
- Equipment: Punch biopsy tool, digital camera, calipers, software for wound area analysis, materials for histology (tissue processor, microtome, stains).
Methodology:
- Wound Creation: Anesthetize animals. Create full-thickness excisional wounds on the dorsum using a sterile punch biopsy tool (e.g., 6-8 mm diameter). Assign animals to treatment groups randomly.
- Treatment Application: Apply the test hydrogel, control hydrogel, or gauze to the wounds. Secure with a secondary transparent film dressing. Change dressings every 2-3 days under anesthesia.
- Macroscopic Analysis:
- Photograph wounds against a scale marker at each dressing change.
- Calculate wound area using image analysis software (e.g., ImageJ).
- Monitor for signs of infection and record observations.
- The primary endpoint is the time to complete wound closure (>95% re-epithelialization).
- Histological Analysis:
- At predetermined time points (e.g., day 7, 14), euthanize animals and harvest the wound tissue with a margin of surrounding skin.
- Process tissues for histology: fix in formalin, embed in paraffin, section, and stain with Hematoxylin and Eosin (H&E) and Masson's Trichrome.
- Perform blinded histological scoring for:
- Re-epithelialization: Percentage of wound covered by new epidermis.
- Granulation Tissue Formation: Thickness and cellularity of new tissue.
- Inflammatory Cell Infiltration: Degree and type of inflammatory response.
- Collagen Deposition: Maturity and organization of collagen fibers (via Trichrome stain).
Signaling Pathways in Wound Healing
Hydrogel dressings are designed to interact with and modulate the complex, overlapping phases of wound healing [18] [33]. The following diagram illustrates the key cellular and molecular events.
Wound Healing Phases and Hydrogel Action
Chronic wounds are often arrested in the inflammatory phase due to factors like biofilm formation and excessive protease activity [18]. Advanced hydrogels are engineered to disrupt this cycle by providing a physical barrier, delivering bioactive molecules, and restoring the optimal healing microenvironment.
The Scientist's Toolkit: Essential Research Reagents and Materials
Table 3: Key Reagents and Materials for Hydrogel Dressing Research
| Item | Function/Application | Examples / Key Characteristics |
|---|---|---|
| Polymer Backbones [18] [33] | Form the primary scaffold of the hydrogel. | Natural: Alginate, Chitosan, Collagen, Hyaluronic Acid, Fibrin. Synthetic: Polyethylene Glycol (PEG), Polyacrylic Acid (PAA), Polyvinyl Alcohol (PVA). |
| Crosslinking Agents [18] [33] | Create 3D network structure; determine mechanical strength and degradation rate. | Ionic crosslinkers (Ca²⺠for alginate), chemical crosslinkers (glutaraldehyde, genipin), photo-initiators (Irgacure 2959 for UV crosslinking). |
| Bioactive Molecules [18] [101] [33] | Impart specific therapeutic functions to the dressing. | Growth Factors: VEGF, FGF, EGF. Antimicrobials: Silver nanoparticles, antimicrobial peptides (AMPs). Anti-inflammatories: Curcumin, corticosteroids. |
| Cell Lines for In Vitro Testing [18] | Assess biocompatibility, cytotoxicity, and cellular responses. | Human Dermal Fibroblasts (HDFs), Human Keratinocytes (HaCaT cell line). |
| Animal Models for In Vivo Testing | Evaluate healing efficacy in a biologically complex system. | Diabetic Models: db/db mice, BKS.Cg-Dock7m +/+ Lepr db/J. Burn Models: Scald or contact burn in rodents. |
| Characterization Equipment | Analyze physical, chemical, and mechanical properties. | Rheometer (mechanical properties), Scanning Electron Microscope (SEM, for morphology), FTIR Spectrometer (chemical structure). |
Conclusion
Hydrogel technology represents a dynamic and rapidly evolving frontier in wound care, successfully transitioning from passive moisture-providing dressings to active, intelligent therapeutic systems. The synthesis of foundational material science with advanced fabrication methods has enabled the creation of multifunctional platforms capable of drug delivery, real-time monitoring, and dynamic interaction with the wound microenvironment. While significant progress is evidenced by positive clinical outcomes—including accelerated healing and effective pain relief—challenges in mechanical optimization, controlled release, and scalable manufacturing remain. The future of hydrogel fabrication lies in the development of fully integrated, closed-loop systems that combine diagnostics and therapeutics, powered by AI and personalized through 3D bioprinting. For researchers and drug developers, the focus must now be on translating these sophisticated laboratory innovations into robust, cost-effective, and accessible clinical solutions to alleviate the global burden of chronic wounds.
References
- 1. Multifunctional self-healing hydrogels for chronic wound ... [https://www.sciencedirect.com/science/article/abs/pii/S0168365925008958]
- 2. Advances in multifunctional diagnostic hydrogels for complex ... [https://pubs.rsc.org/en/content/articlehtml/2025/sd/d5sd00051c]
- 3. A dynamically phase-adaptive regulating hydrogel ... [https://www.nature.com/articles/s41467-025-58987-w]
- 4. Review Hydrogel-based dressing for wound healing [https://www.sciencedirect.com/science/article/abs/pii/S0141813025028740]
- 5. Innovations in Designing Hydrogels for Advanced Wound ... [https://www.mdpi.com/2310-2861/11/5/332]
- 6. Towards Intelligent Wound Care: Hydrogel-Based ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12252240/]
- 7. Selfâ€Healing Hydrogels: Mechanisms and Biomedical ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12018771/]
- 8. The Role of Natural Hydrogels in Enhancing Wound Healing [https://pmc.ncbi.nlm.nih.gov/articles/PMC12566826/]
- 9. Development of hyaluronic acid-based hydrogels for ... [https://www.sciencedirect.com/science/article/abs/pii/S0141813025028259]
- 10. A Recent Insight into Research Pertaining to Collagen ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12295635/]
- 11. Next-Generation Wound Healing Materials: Role of ... [https://www.mdpi.com/2073-4360/17/16/2244]
- 12. Recent Advances on Chitosan-Based Thermosensitive ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12189897/]
- 13. Advanced wound healing with chitosan hydrogels ... [https://www.frontiersin.org/journals/materials/articles/10.3389/fmats.2025.1573222/full]
- 14. How the combination of alginate and chitosan can fabricate ... [https://www.sciencedirect.com/science/article/pii/S240584402408071X]
- 15. Hyaluronic acid-based hybrid hydrogel synergizes ... [https://pubmed.ncbi.nlm.nih.gov/41218676/]
- 16. Alginate-based hydrogels for sustained antimicrobial ... [https://www.sciencedirect.com/science/article/pii/S2772950825001645]
- 17. Functional Properties of Gelatin–Alginate Hydrogels for ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC11941921/]
- 18. Hydrogels and Wound Healing: Current and Future ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC10815795/]
- 19. Synthesis of PVA-Based Hydrogels for Biomedical Applications [https://pmc.ncbi.nlm.nih.gov/articles/PMC11854265/]
- 20. PVA-CO-AAM and peg-co-aam hydrogels as bromelain ... [https://www.sciencedirect.com/science/article/abs/pii/S1773224721001635]
- 21. Comparison of Wound Healing Effects of Different Micro- ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12026865/]
- 22. Construction methods and biomedical applications of PVA ... [https://www.frontiersin.org/journals/chemistry/articles/10.3389/fchem.2024.1376799/full]
- 23. Physically Cross-Linked PVA Hydrogels as Potential ... [https://www.mdpi.com/1999-4923/16/11/1388]
- 24. Fabrication of polyvinyl alcohol-polyethylene glycol-copper ... [https://pubmed.ncbi.nlm.nih.gov/40974566/]
- 25. A Bi-Layer PVA/CMC/PEG Hydrogel with Gradually ... [https://pubmed.ncbi.nlm.nih.gov/30840367/]
- 26. Fabrication of pH-stimuli hydrogel as bioactive materials for ... [https://www.sciencedirect.com/science/article/pii/S2405844024088959]
- 27. Biological Models for Evaluating Hydrogel-Based ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12469554/]
- 28. Preparation of Hydrogel by Crosslinking and Multi- ... [https://www.mdpi.com/2310-2861/11/11/896]
- 29. Hydrogel crosslinking modulates macrophages, fibroblasts, ... [https://www.nature.com/articles/s41467-024-50072-y]
- 30. Enhancing wound healing with nanoparticle-infused ... [https://link.springer.com/article/10.1007/s44340-025-00030-1]
- 31. using hydrogel crosslinking chemistry to tune release of micro ... [https://pubs.rsc.org/en/content/articlehtml/2025/pm/d4pm00186a]
- 32. Advancements in Hydrogels - PubMed Central - NIH [https://pmc.ncbi.nlm.nih.gov/articles/PMC12349326/]
- 33. Innovative hydrogels in cutaneous wound healing [https://www.frontiersin.org/journals/bioengineering-and-biotechnology/articles/10.3389/fbioe.2025.1454903/full]
- 34. a next generation biomaterial for enhanced wound healing [https://pubs.rsc.org/en/content/articlehtml/2025/bm/d5bm00095e]
- 35. Applications of hydrogels in wound healing: A narrative ... [https://www.jnursrcp.com/article_202312.html]
- 36. Advancements in hydrogels: A comprehensive review of ... [https://www.sciencedirect.com/science/article/abs/pii/S0141813025078274]
- 37. Advancements in Hydrogels: A Comprehensive Review of ... [https://www.mdpi.com/2073-4360/17/15/2026]
- 38. Light-Mediated 3D-Printed Wound Dressings Based on ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12031436/]
- 39. 3D printed antibacterial and anti-inflammatory scaffold ... [https://www.nature.com/articles/s41598-025-18174-9]
- 40. Electrospinning in promoting chronic wound healing [https://www.frontiersin.org/journals/bioengineering-and-biotechnology/articles/10.3389/fbioe.2025.1550553/full]
- 41. Advancements in Portable Electrospinning Technology for ... [https://link.springer.com/article/10.1007/s44174-025-00409-y]
- 42. Microenvironment-feedback regulated hydrogels as living ... [https://www.nature.com/articles/s41467-025-60858-3]
- 43. Advances in Composite Stimuli-Responsive Hydrogels for ... [https://www.mdpi.com/2310-2861/11/6/420]
- 44. Hydrogel Preparation Methods and Biomaterials for Wound ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC8540918/]
- 45. Stimuli-responsive hydrogels for skin wound healing and ... [https://link.springer.com/article/10.1007/s42247-024-00930-8]
- 46. Rational Design of Self-Healing Hydrogel with High ... [https://www.mdpi.com/2310-2861/11/10/807]
- 47. Unlocking the potential of stimuli-responsive injectable ... [https://www.frontiersin.org/journals/biomaterials-science/articles/10.3389/fbiom.2025.1641339/full]
- 48. Stiff and self-healing hydrogels by polymer entanglements ... [https://www.nature.com/articles/s41563-025-02146-5]
- 49. 3D-printed hyaluronic acid-based hydrogel in wound ... [https://www.sciencedirect.com/science/article/abs/pii/S0014305725004288]
- 50. Hydrogel delivering self-assembled herbal nanoparticles ... [https://pubmed.ncbi.nlm.nih.gov/41169791/]
- 51. Hydrogel Delivering Self-Assembled Herbal Nanoparticles ... [https://www.sciencedirect.com/science/article/pii/S2590006425009883]
- 52. The Hidden Power of the Secretome: Therapeutic Potential on ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC11899803/]
- 53. Smart Hydrogels in Wearable Electronics for Wound Treatments [https://pmc.ncbi.nlm.nih.gov/articles/PMC12614155/]
- 54. Prospects of Hydrogels in Wound Healing: Toward the ... [https://www.mdpi.com/1999-4923/17/11/1486]
- 55. Towards Intelligent Wound Care: Hydrogel-Based ... [https://www.mdpi.com/2073-4360/17/13/1881]
- 56. Facile fabrication of biomimetic and conductive hydrogels ... [https://www.sciencedirect.com/science/article/abs/pii/S1385894725019333]
- 57. Hydrogels for Translucent Wearable Electronics: Innovations ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12111672/]
- 58. Advanced healing potential of simple natural hydrogel ... [https://www.sciencedirect.com/science/article/pii/S2590156725000131]
- 59. Towards adaptive bioelectronic wound therapy with ... [https://www.nature.com/articles/s44385-025-00038-6]
- 60. Innovations in hydrogel therapies for diabetic wound healing [https://pmc.ncbi.nlm.nih.gov/articles/PMC12309386/]
- 61. Effects of Carbonâ€Based and Organic Nanoparticles in ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12214411/]
- 62. Wound healing properties and antibiofilm activity of ... [https://www.nature.com/articles/s41598-025-12385-w]
- 63. Next-Generation Hydrogel Design: Computational ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12115241/]
- 64. Types of Hydrogels to Deliver Cells for Wound Healing [https://bio-protocol.org/exchange/minidetail?id=9620514&type=30]
- 65. Porous hierarchically ordered hydrogels demonstrating ... [https://www.nature.com/articles/s41467-025-59171-w]
- 66. Review Gradient hydrogels: From fabrication strategies ... [https://www.sciencedirect.com/science/article/abs/pii/S1385894725082294]
- 67. Biomaterials-mediated sequential drug delivery: emerging ... [https://www.sciencedirect.com/science/article/pii/S1818087625000728]
- 68. Hydrogel Conjugation: Engineering of Hydrogels for Drug ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12300585/]
- 69. Recent advances and challenges of injectable hydrogels in ... [https://www.sciencedirect.com/science/article/abs/pii/S016836592500642X]
- 70. Multifunctional Nanomaterial-Integrated Hydrogels for ... [https://www.mdpi.com/2310-2861/11/11/892]
- 71. Smart hydrogels for in situ tissue drug delivery [https://jbiomedsci.biomedcentral.com/articles/10.1186/s12929-025-01166-2]
- 72. Hydrogels for Wound Dressings: Applications in Burn ... [https://www.mdpi.com/2504-477X/9/3/133]
- 73. Antimicrobial hydrogels for the treatment of infection - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC3929057/]
- 74. Advances in preparation and application of antibacterial ... [https://jnanobiotechnology.biomedcentral.com/articles/10.1186/s12951-023-02025-8]
- 75. Innovations in Designing Hydrogels for Advanced Wound ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12111174/]
- 76. Antimicrobial Natural Hydrogels in Biomedicine: Properties ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC9917233/]
- 77. Enhanced antibacterial activity of porous chitosan-based ... [https://www.nature.com/articles/s41598-024-58174-9]
- 78. Anti-inflammatory and antibacterial hydrogel based on a ... [https://www.sciencedirect.com/science/article/pii/S1742706125001783]
- 79. Emerging Fabrication Strategies of Hydrogels and Its ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC9024659/]
- 80. Tailoring self-assembled granular hydrogels (GHs) for... [https://the-innovation.org/article/doi/10.59717/j.xinn-mater.2025.100154]
- 81. What Are the Challenges in Scaling Injectable Hydrogel Production [https://eureka.patsnap.com/report-challenges-in-scaling-injectable-hydrogel-production]
- 82. Hydrogel fabrication using emerging technologies [https://www.sciencedirect.com/science/article/abs/pii/S1466856425003108]
- 83. Hydrogel Films in Biomedical Applications: Fabrication, ... [https://www.mdpi.com/2310-2861/11/11/918]
- 84. Hydrogel Market Size, Share | Forecast Report [2025-2032] [https://www.fortunebusinessinsights.com/hydrogel-market-109679]
- 85. | Abcam Wound healing assay [https://www.abcam.com/en-us/technical-resources/protocols/wound-healing-assay]
- 86. SDF-1α loaded lipid liquid crystalline hydrogel accelerates ... [https://www.nature.com/articles/s41598-025-10756-x]
- 87. Modeling Wound Chronicity In Vivo: The Translational ... [https://www.sciencedirect.com/science/article/pii/S0022202X2400006X]
- 88. Modeling epithelial wound closure dynamics with AI [https://pmc.ncbi.nlm.nih.gov/articles/PMC12528918/]
- 89. Enhancing chronic wound assessment through agreement ... [https://www.nature.com/articles/s41598-025-06703-5]
- 90. Comparative efficacy of different functional hydrogel ... [https://pubmed.ncbi.nlm.nih.gov/40197692/]
- 91. The Wound Reporting in Animal and Human Preclinical ... [https://pubmed.ncbi.nlm.nih.gov/39639458/?utm_source=SimplePie&utm_medium=rss&utm_campaign=pubmed-2&utm_content=1DqqIY7i9Icf9J_BIwh0PRg_lq4ZdpZVa2NCCBt_Z5SBUCUY-a&fc=20220524054013&ff=20241206091007&v=2.18.0.post9+e462414]
- 92. Frontiers | A Systematic Review and Meta-Analysis of Clinical Effectiveness and Safety of Hydrogel Dressings in the Management of Skin Wounds [https://www.frontiersin.org/journals/bioengineering-and-biotechnology/articles/10.3389/fbioe.2019.00342/full]
- 93. A Systematic Review and Meta-Analysis of Clinical ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC6881259/]
- 94. Modern Wound Dressings: Hydrogel Dressings - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC8472341/]
- 95. Wound Dressings and Comparative Effectiveness Data - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC4121107/]
- 96. Hydrogel-based dressing for wound healing: A systematic ... [https://pubmed.ncbi.nlm.nih.gov/40118421/]
- 97. A practical guide to hydrogels for cell culture [https://www.nature.com/articles/nmeth.3839]
- 98. Hydrogel Wound Dressing Trends and Forecast 2025-2033 [https://www.datainsightsmarket.com/reports/hydrogel-wound-dressing-952101]
- 99. Hydrogel Dressing Market to Reach USD 1.29 Billion by ... [https://www.globenewswire.com/news-release/2025/09/30/3158696/0/en/Hydrogel-Dressing-Market-to-Reach-USD-1-29-Billion-by-2032-as-U-S-Demand-Surges-for-Advanced-Wound-Care-S-S-Insider.html]
- 100. Hydrogel Dressing Market Size, Share, Growth [https://www.fortunebusinessinsights.com/hydrogel-dressing-market-106252]
- 101. Hydrogel Dressing Market Size & Share 2025-2032 [https://www.360iresearch.com/library/intelligence/hydrogel-dressing]
- 102. Hydrogel Dressing Market - Global Forecast 2025-2032 [https://www.researchandmarkets.com/reports/5716178/hydrogel-dressing-market-global-forecast-2025?srsltid=AfmBOoqflhtDYdXlwefLa944IRvM9BE6_U9iPckUm4AsyaZZndNLQ-L1]